Medical Network April 8th On April 4th, Buchang Pharmaceutical announced that the wholly-owned subsidiary Danhong Pharmaceutical has developed a recombinant human anti-tumor necrosis factor-α (TNF-α) full-human monoclonal antibody injection. "Through the approval of the clinical trial ethics of the Medical Ethics Committee of Jinan Central Hospital, the ethical examination approval document was obtained, and the phase I clinical trial was officially launched.
Basic situation of drugs

The BC002 project is a monoclonal antibody drug developed by Shandong Danhong Pharmaceutical Co., Ltd., which is a biosimilar drug of Aibowei adalimumab injection (trade name: Xiumee), which belongs to the category of therapeutic biological products. The clinical indications for use are autoimmune diseases such as rheumatoid arthritis.
Adalimumab injection is the world's first approved whole-human anti- tumor necrosis factor (TNF-α) monoclonal antibody developed and approved by Abbott. It is mainly used to treat rheumatoid arthritis, ankylosing spondylitis and Autoimmune diseases such as psoriasis, which rose rapidly after being approved for marketing in the United States in 2002, has consistently dominated the global drug sales list for nearly seven years. According to Aberdeen’s earnings report, Xiu Meile’s global sales in 2018 were US$19.936 billion.
   Global sales of adalimumab (unit: billion US dollars)
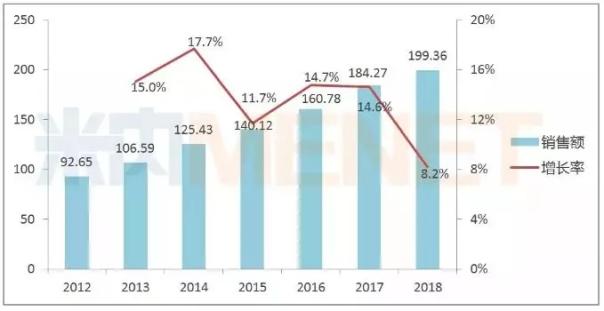
In February 2010, adalimumab injection was approved for marketing in China, and its sales have been flat for many years. According to the data of the intranet, the sales of the terminal repairing beauty of China's public medical institutions in 2017 was 81.25 million yuan, and the huge domestic market space has not yet been tapped.
At present, there are nearly 30 domestic companies that have researched and developed this product. The listed pharmaceutical companies such as Hisun Pharmaceutical, Fosun Pharma , and Hualan Bio are also optimistic about the market, and are in the process of promoting the development of similar drugs for adalimumab. Stephan Pharmaceutical said it will strive to adjust its product development strategy and indications, accelerate the progress of clinical trials, and minimize the competition risk of similar varieties.
As of March 31, 2019, the company's research and development expenses for the BC002 project amounted to approximately RMB 24,698,800. Previously, Buchang Pharmaceutical released a biopharmaceutical strategic plan. The company said that it will transform from a localized enterprise to a globalized enterprise, and strive to build a whole bio-pharmaceutical enterprise's entire industrial chain and enhance the research and development capabilities of biopharmaceuticals.
   Data source: Minenet database, company announcement
X Ray Straight Lead Neck Protection,Lead Neck Collar,X Ray Thyroid Shield Collar,Radiation Thyroid Shields Collar
Longkou Kangxie Medical Instrument Co., Ltd , https://www.sdkangxiemedical.com