The biomedical industry is composed of the biotechnology industry and the pharmaceutical industry. Bio-innovative drugs are emerging industries in the pharmaceutical industry. The national “Twelfth Five-Year Plan†has identified the focus of biomedical development, including monoclonal antibody cloning drugs, protein drugs, genes and nucleic acid drugs, etc. Excellent policies will actively promote biomedicine in China. With rapid development, the biomedical industry is expected. Among them, monoclonal antibody drugs, as a biologically targeted drug with unique advantages, have the characteristics of high specificity, strong targeting and low toxic and side effects, and have significant effects in treatment. With the continuous development of antibody technology and the emergence of new antibodies, monoclonal antibody drugs have become one of the fastest growing areas in the pharmaceutical industry. One-quarter of the biotech drugs currently under study are monoclonal antibody drugs. Various monoclonal antibody derivatives have emerged during this period, including antibody drug conjugates, small molecule antibodies, and bispecific antibodies. The domestic monoclonal antibody field faces the dual opportunities of rapid market growth and import substitution. Monoclonal antibody research has been included in the 863 Program and national key research projects. The research, development and market application of monoclonal antibody drugs will certainly attract the participation and layout of a large number of pharmaceutical companies. With this shareholder wind, there will be autonomous monoclonal antibodies in the future. Class dominance should not be just a dream
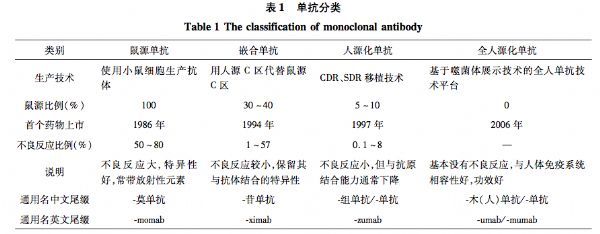
I. Definition and classification of monoclonal antibodies
A highly homologous antibody produced by a single B cell clone, directed only to a particular epitope, is referred to as a monoclonal antibody. Traditionally, hybridoma technology has been used. Hybridoma antibody technology is based on cell fusion technology, and sensitized B cells with the ability to secrete specific antibodies and myeloma cells with unlimited reproduction ability are fused into B cell hybridomas. By culturing a cell population with a single hybridoma cell having such characteristics, a characteristic antibody against an epitope can be prepared, that is, a monoclonal antibody.
Through genetic engineering, the antibody molecules in the monoclonal antibody and the human body have as similar characteristics as possible, and the antibody is humanized. From the murine source to the whole source, the probability of human anti-mouse immune response in the patient is gradually reduced, and the therapeutic effect and safety are gradually improved. Therefore, the whole human monoclonal antibody is the trend of development of monoclonal antibody (Table 1). However, as the degree of humanization increases, the affinity of monoclonal antibody products will gradually decline. Therefore, improving the affinity of monoclonal antibody products under the premise of eliminating human anti-mouse response is the core technical barrier for the development of monoclonal antibody drugs.
Second, monoclonal antibody drug development process and trends
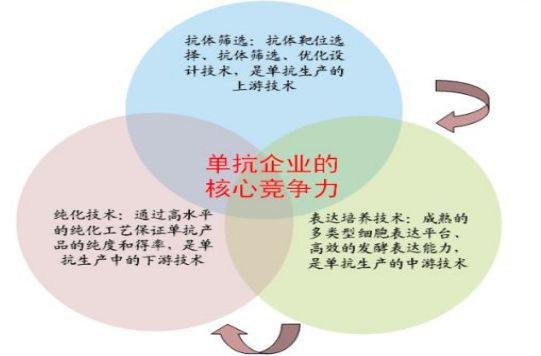
In general, a monoclonal antibody drug company needs an upstream R&D platform to screen out the ideal monoclonal antibody; and it has a high-efficiency and large-scale fermentation to express the monoclonal antibody; it also needs to be able to separate and purify the monoclonal antibody with high efficiency and high purity. Such a company has the core competitiveness of the development and production of monoclonal antibodies.
1. Phage display technology has become the mainstream of human monoclonal antibody screening. With the deepening of the humanization process of monoclonal antibodies, phage display technology (using PCR technology to amplify the entire set of antibody heavy chain variable region (VH) and light chain variable region (VL) genes from human immune cells, Large-scale monoclonal antibody screening platforms that are cloned into phage vectors and expressed in the form of fusion proteins on the surface of their outer shells are gaining increasing attention. The technology can not only obtain the whole human monoclonal antibody, but also does not require cell fusion, the test period is short, and the process is simple. It is a major breakthrough in the preparation technology of human antibody. At present, the mainstream domestic monoclonal antibody manufacturers use phage display technology to screen the monoclonal antibody.
2. Expression culture technology is the key to yield formation and quality control of monoclonal antibody. Expression method, reactor size, expression system and expression level are important indicators for judging the technical level of enterprises.
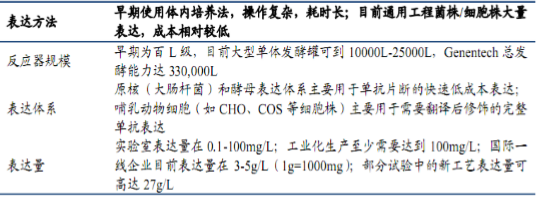
Table 2 Three elements of expression culture technology
Analog Hearing Aid
Analog Hearing Aid
Shenzhen Sunshine Technology Co.,Ltd , https://www.shenzhenyatwin.com