Medical Network January 10th In 2018, the US FDA approved the listing of 59 new molecular entities, breaking the record of silence for many years; in China, the State Food and Drug Administration (NMPA) also approved 48 new drugs on the market as never before. Of the 48 new drugs, 38 are imported and 10 are domestically produced.
It is worth mentioning that 9 of the 10 domestically produced drugs are the first new molecules approved in the world – surpassing EMA (European Drug Administration) and PMDA (Japan Pharmaceutical and Medical Devices Bureau) for the first time in quantity.
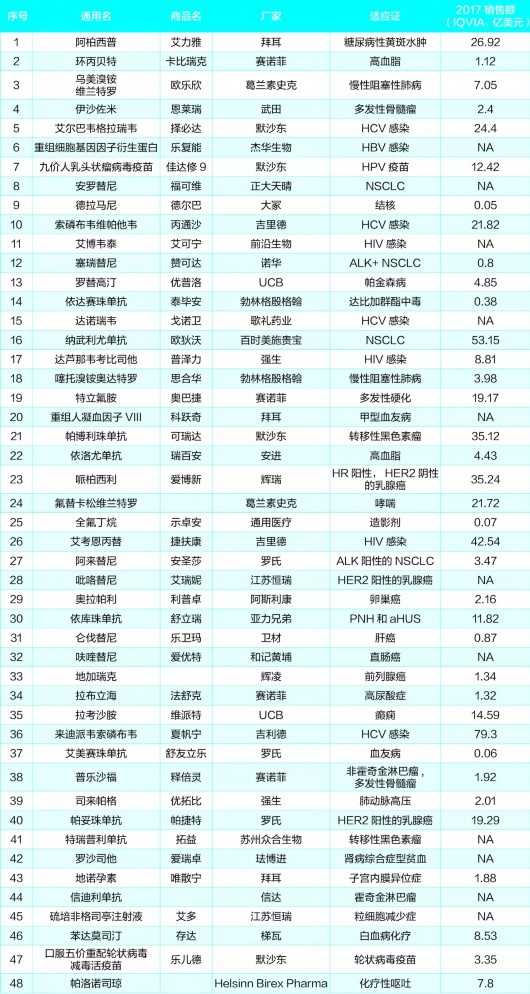
  List of new drugs approved in 2018
From the point of view of indications, the new drugs approved by the State Food and Drug Administration in 2018 are mostly anticancer drugs, antiviral drugs and orphan drugs.
Antineoplastic agents
Tumors are life-threatening diseases. In China, there are nearly 4 million new cases each year and 2.3 million deaths. Antineoplastic drugs have enormous clinical needs.
Based on the author's statistics, 18 of the 48 new drugs approved by the State Food and Drug Administration in 2018 are anti-tumor drugs, and the indications involved are:
Multiple myeloma (MM), non-small cell lung cancer (NSCLC), cervical cancer, ovarian cancer, breast cancer, melanoma, liver cancer, rectal cancer, prostate cancer, leukemia and lymphoma.
A number of other products are also associated with tumor indications, such as palonosetron approved for the treatment of chemotherapy-induced vomiting, and Labrite is approved for the treatment of childhood leukemia and uric acid levels in patients with lymphoma. The listing of these products will greatly enrich the choice of cancer treatment in China, and largely change the current status of treatment with chemotherapy as the mainstream.
Of the 18 anticancer drugs, 13 are imported new drugs. Among them, some products have developed into a heavy bomb in just a few years because of the great advantage of curative effect (the sales in the 5 years after the listing exceeded 1 billion US dollars), such as Pfizer's Pipersili, Bristol-Myers Squibb's Navuli Yudan and Merck's Pabolizumab.
Some products, although not up to the heavy "bomb" level of sales, have revolutionized their efficacy, such as the anaplastic lymphoma kinase (ALK) inhibitor alittinib, which can significantly prolong ALK-positive NSCLC patients. Progression-free survival (PFS), median PFS more than doubled compared with chemotherapy (16.6 months vs 8.1 months); poly ADP ribose polymerase (PARP) inhibitor olalapril, can significantly prolong BRCA mutation ovarian cancer patients Progression free survival (19.1 months vs 5.5 months).
In addition to prolonging the survival period, the patient's benefit also includes a significant improvement in the quality of life. Many cancer patients are under the control of targeted drugs, and their mental state is no different from ordinary people.
Antiviral drugs
Although China is a major consumer of antibiotics, China's antiviral drugs are very scarce. China is a large country of hepatitis C, with 7.6 million hepatitis C virus infections, and the treatment needs are huge.
In addition to hepatitis C, the biggest breakthrough in international antiviral treatment is AIDS. There are 1.25 million HIV-infected people in China, and the demand for treatment is huge. However, due to many historical factors, China's AIDS treatment market is very “coolâ€, and the treatment plan lags behind developed countries and regions in Europe and America.
Hepatitis C and AIDS are major diseases that threaten people's health . To this end, the State Food and Drug Administration has listed drugs for treating AIDS and hepatitis C as the key targets for care. After a priority review, starting from the end of 2017, multiple unilateral hepatitis C. Special effects drugs were approved for listing.
Since 2018, the international heavy hepatitis C cocktail Albave gragrevir tablets, sophophos-bivevivirapoxi tablets and Ladi Weiweisuo Phosphorus tablets have been approved, so that China's hepatitis C treatment has crossed the second The generation (NS4/3 protease inhibitor + interferon) and the third generation (sofobuti + ribavirin) therapy directly entered the cocktail era.
In terms of AIDS drugs, the State Food and Drug Administration approved the launch of the original indigenous innovative drug Aiboweitai and imported cocktails, acecolide tablets, emtricitabine propofol and darunavir tablets. The accessibility of AIDS drugs in China has been greatly improved.
In addition to drugs for the treatment of hepatitis C and AIDS, the new antiviral drugs approved in 2018 include a new generation of drugs for the treatment of hepatitis B, propofol fortuneus, and domestic hepatitis B new drug recombinant cell gene factor-derived proteins. The listing of these drugs will benefit the majority of hepatitis B patients. .
Anti-asthma and COPD drugs
According to statistics, there are about 334 million people with asthma worldwide, about 328 million people with chronic obstructive pulmonary disease (COPD), 3.2 million people die of COPD every year, and 400,000 die of asthma. COPD And asthma is even a more terrible disease than lung cancer.
The incidence of COPD in China is 8.6%, and the total number of patients is 99.9 million. Asthma, according to the 2014 epidemiological survey, there are about 25 million to 30 million asthma patients in China. Asthma and COPD seriously affect the quality of life of our people.
The huge drug market has made inhalants as the preferred treatment for asthma and COPD a target for domestic pharmaceutical companies and multinational pharmaceutical companies.
The combined mortality rate of asthma and COPD exceeds that of lung cancer, and the treatment needs are enormous. But compared to the United States, China's treatment plan is still single, small products, the market is highly concentrated in AstraZeneca led by multinational enterprises.
In 2018, the State Food and Drug Administration approved Boehringer Ingelheim's Tiotropium Odaro Inhaler and Odatrol Inhaler, GlaxoSmithKline's Ume Bromide Virantro Inhaler and Fluticasone Rantero inhalers, all of which are the latest generation of therapies, have been on the market for more than three years, and the clinical evidence has been sufficient. In 2017, total global sales reached $3.3 billion. The launch of these products can significantly enrich the clinical treatment options for asthma and COPD, and is expected to change the status quo of "one big" in the asthma and COPD treatment market.
Orphan drug
In recent years, orphan drugs have become a huge driving force for the growth of the international innovative medicine market. However, compared with developed countries and regions in Europe and America, the accessibility of orphan drugs in China is still relatively low, and many patients with rare diseases have not been effectively treated.
Many drugs approved by the State Food and Drug Administration in 2018 were classified as orphan drugs by the US FDA under the same indications, including treatment of ALK-positive non-small cell lung cancer drugs alittinib and seratinib for the treatment of melanoma. Pabolizumab, a drug for the treatment of non-Hodgkin's lymphoma, proxafu, a leukemia chemotherapeutic drug, bendamustine, a drug for the treatment of multiple sclerosis, and a hemophilia drug, Yueqi (recombinant clotting factor) VIII) with Emeraldizumab, a drug for the treatment of hyperuricemia, Rabli, a drug for the treatment of pulmonary hypertension, selepa, paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome Syndrome (aHUS) drug etumazumab, etc., the listing of these drugs will greatly improve the treatment level of rare diseases in China.
Domestic new drug
In 2018, the State Food and Drug Administration approved nine independent innovative drugs, including Hengrui's pyrrolidine and thiophene statin, Zhengtaiqing's erlotinib, and Hutchison's furazolinib. Bio-Trepril monoclonal antibody, Cinda's Cindy monoclonal antibody, Danolivi of Gyula Pharmaceuticals, Aiboweitai of the frontier organism, and recombinant cellular gene factor-derived proteins of Jiehua Bio.
The listing of these products shows that China has made great achievements in encouraging and guiding innovation policies. On the other hand, it also shows that China's innovation ability and level still need to be improved, because these products are still dominated by "Me-Too" innovative drugs. And the repeat layout is more serious. The data shows that there are currently 40 PD-1 antibodies in the development stage in China.
In addition to the nine independent innovation drugs, the new drug approved by the State Food and Drug Administration for the first time is Rosita, which is a “First-in-class†drug.
As of now, the global multi-center clinical trial results of this product have reached the end of treatment, and it is expected to be approved by the FDA and EMA in 2019. The author believes that the State Food and Drug Administration approved the "First-in-class" innovative drug market before the FDA and EMA is unprecedented. Once this historic step is taken, it will become a normal state in the future.
Innovative drugs account for more than 70% of the global pharmaceutical market and are a major source of profit for the pharmaceutical industry. The author believes that with the advancement of drug procurement, in the next few years, generic drugs will no longer be the main growth point of China's pharmaceutical market. Enterprises must be active and innovative if they want to become bigger and stronger.
According to the data reported by the European Union Pharmaceutical Industry Association, 64.7% of the new drugs listed in 2011-2016 were from the United States. Therefore, Chinese pharmaceutical companies must not only actively innovate, but also actively go out. Although after the 21st century, the FDA has tightened the evaluation scale of “Me-Too†drugs, some high-quality domestic “Me-Too†innovative drugs are also expected to break through national borders and move from “China’s newâ€. "The world is new."
In 2008, 48 new drugs were approved for marketing, and the accessibility of many indication drugs will be greatly improved. However, the improvement of accessibility only realizes the change from “no medicine available†to “medical availabilityâ€. To truly benefit the majority of patients, it is necessary to realize the transition from “available medicine†to “medical and affordableâ€. .
Tuna Loin,Frozen Tuna Loin,Fresh Tuna Loin,Yellowfin Tuna Loin
ZHEJIANG RETRONX FOODSTUFF INDUSTRY CO.,LTD , https://www.retronxfoods.com