Today, flow cytometry has become a powerful technology for analyzing multiple parameters of thousands of individual cells or other particles in seconds. In flow cytometry, after the fluorescein is excited by a certain wavelength of laser, the released energy can emit a certain wavelength of fluorescence. Therefore, we should pay attention to their excitation wavelength and emission wavelength when choosing fluorescein to choose the correct one. Laser and fluorescence combination. With the advent of multi-color flow cytometry, new fluorescein and labeled antibodies have also been developed. However, choosing the best combination of fluorescent dyes for the antibodies required in the experiment is a complex process. Elabscience's technical team summarizes the selection criteria for fluorescein in some commonly used flow fluorescein and multicolor flow cytometry to help you avoid repetitive experiments and errors and increase your experiment success rate:
1. Type characteristics and detection filters of commonly used fluorescein
Faced with a wide variety of fluorescent dyes, how do you know if these dyes are suitable for your flow cytometer and how much color compensation is between these dyes?
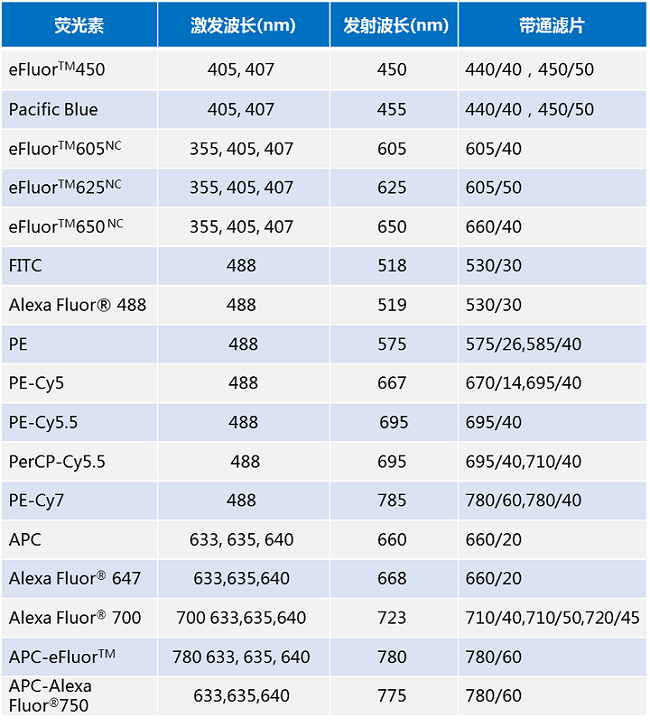
Note:
a. Take eFluorTM450 as an example: the maximum excitation wavelength is 405 nm or 407 nm, and the maximum emission wavelength is 450 nm (the maximum excitation wavelength depends on the excitation light of the flow cytometer laser), which requires 440/40 (ie, the detection wavelength range is 440). Bandpass filter (BP, Band Pass) of ±20 nm) or 450/50 (ie, detection wavelength range of 440±25 nm).
b. Take eFluorTM 605NC as an example: the maximum excitation wavelength is 335 nm, 405 nm or 407 nm, the maximum emission wavelength is 605 nm, and the flow cytometry requires 605/40 (ie, the detection wavelength range is 605±20 nm). Pass the filter.
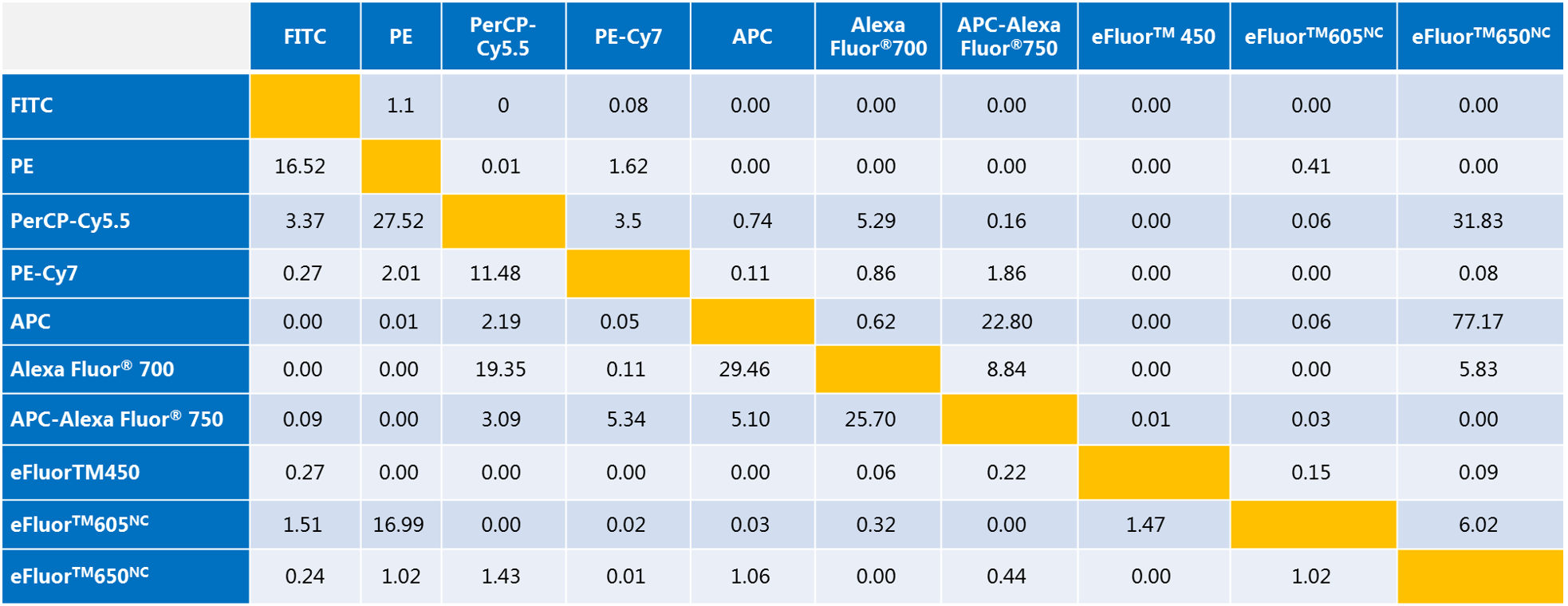
Note:
a. The values ​​in the table indicate the color compensation values ​​between the fluorescence detection channels in %.
b. Take FITC and PE as an example: If the FL1 channel of the flow cytometer detects FITC and the FL2 channel detects PE, the compensation of the instrument should be set to FL1-%FL2=1.10, FL2-%FL1=16.52. It is understandable 1.10% of PE fluorescein leaks into the FL1 detection channel and needs to be subtracted from FL1, while 16.52% of FITC fluorescein leaks into the FL2 detection channel and needs to be subtracted from FL2.
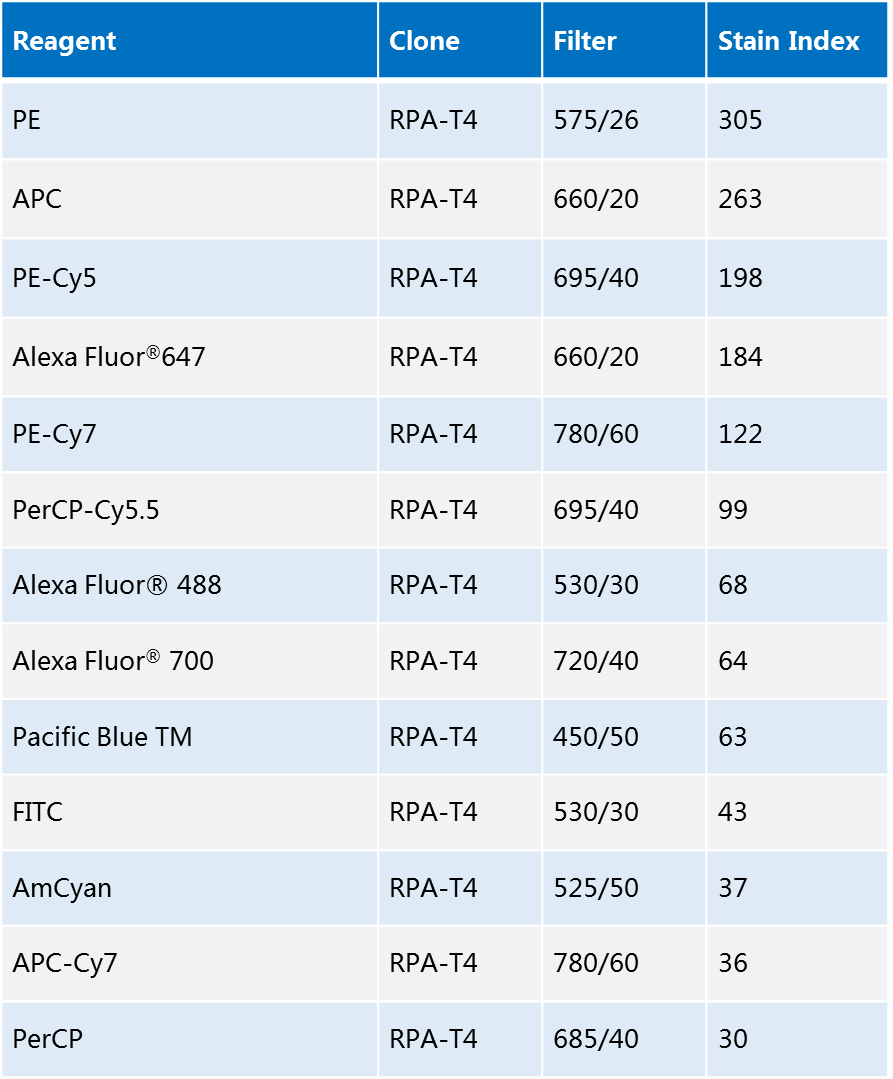
2. Comparison of common fluorescein fluorescence intensities
The intensity of fluorescence is related to the staining index (Stain Index). The higher the dyeing index, the better the fluorescence intensity; the dyeing index is lower and the staining intensity is relatively poor.
The intensity of each fluorescein can be seen by the dyeing index: PE>APC>FITC>PerCP
3. Principles of fluorescein selection in multicolor flow cytometry
1) Select fluorescein according to the machine configuration
Know the performance of the flow cytometer you use. Most flow cytometers have two or more lasers and are available in five wavelengths: UV (355 nm), Purple (405 nm), Blue (488 nm). ), yellow (561 nm) and red (640 nm). In addition to the laser, the specific laser chip and detector also determine how many colors your configuration can detect at the same time.
Multi-color labeling fluorescein matching principle: only one fluorescein can be selected per channel; the fluorescein between each channel can be arbitrarily matched. Commonly used four-color combinations are: FITC, PE, PerCP, APC.
2) Try to choose a fluorescent substance with high fluorescence
FITC, PE, PC-CY5 and APC are currently commonly used fluorescein for flow cytometry, which can basically meet the needs of daily clinical and scientific research, and try to choose fluorescein with strong fluorescence. The expression levels of various antigens on the surface or inside the cells are also different. For example, CD4 and CD8 have many expression molecules on the cell surface, while molecules such as CD25 are not expressed on the cells. When analyzing these molecules by flow cytometry, Fluorescein should be selected according to the expression level. For molecules with a large expression amount, weak fluorescein such as FITC can be used, and for molecules with a small expression amount, a fluorescent substance with strong fluorescence should be selected for your specific instrument configuration, such as PE, PE-CY5 or PE-CY5.5.
3) Ensure that the overlap of the quality inspection emission spectra of fluorescein is as small as possible
When the emission spectra of the two fluorophores overlap, it may be observed that one fluorophore leaks into the other detection channel (such as FITC and PE). If fluorescence compensation techniques are not applied, it is difficult to distinguish between different groups. It is best to choose few or no overlapping fluorophores to minimize this leakage. Choosing a combination of reagents minimizes the possibility of spectral superposition, which may conflict with the rules for selecting the brightest fluorescein. For example, in a four-color analysis, PE-Cy5-labeled antibodies are often paired with APC-labeled antibodies. Use, this combination requires a large adjustment of the color compensation of both. PE-Cy5.5 can be used instead of PE-Cy5, and the color compensation is only about 1%. Because the excitation wavelength of Cy5 is close to the excitation wavelength of APC, Cy5 in PE-Cy5 fluorescein is also excited by the second laser (氦氖-laser) that excites APC, resulting in very good color compensation for PE-Cy5 and APC. Hard to adjust. In contrast, the Cy5.5 excitation wavelength in PE-Cy5.5 is 675 nm, which cannot be excited by the second laser that excites APC. There is no interference from Cy5.5 to APC, and the color compensation is small. Therefore, it may be necessary to sacrifice the brightness of individual fluoresceins to avoid fluorescence leakage and loss of sensitivity.
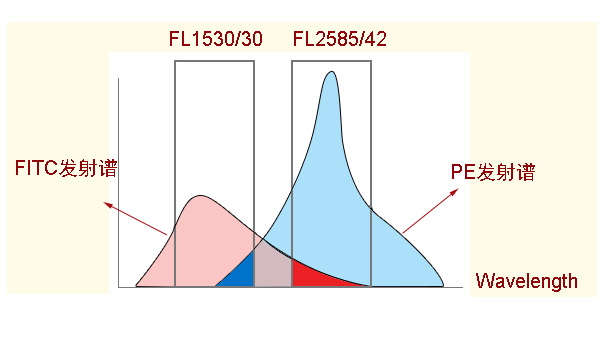
To adjust the compensation by single standard comparison:
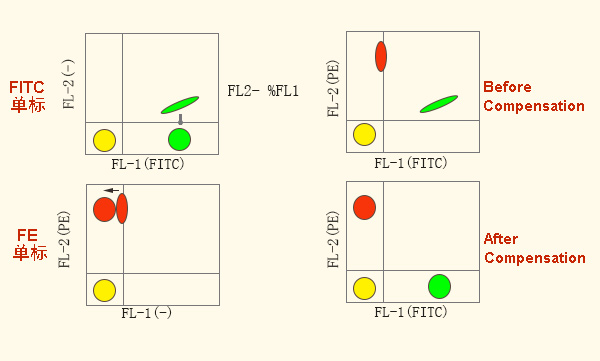
Therefore, try to choose fluorescein with small spectral overlap, such as FITC/PE-Cy7; choose different fluorescein excited by laser, such as FITC/APC, PE/APC.

4) Try to avoid false positives caused by the use of coupled fluorescent dyes
As the number of colors continues to increase, coupled fluorescent dyes may be used in the experiment. Coupling a fluorescent dye means that two fluoresceins are linked together for fluorescence resonance energy transfer, such as PE-Alexa-Fluor 647 or APC-Cy7. However, the coupled fluorescent dye is easily degraded, resulting in uncoupling and fluorescence emission at two wavelengths, resulting in false positives. If coupled fluorescein is used, it is necessary to complete the experiment as soon as possible in a short time, and the longer the exposure time, The more unstable fluorescein is. During fixation and permeabilization, the brightness of the coupled fluorescent dye may be reduced, so the procedure should be as gentle as possible. The compensation value can be obtained by a sample processed in the same manner.
Foot Spa Massager,Bath Foot Massager Machine,Foot Spa Bath,Bath Foot Massager With Bubble
Huaian Mimir Electric Appliance Co., LTD , https://www.mmfootbath.com