Chemiluminescent Cell Experiment of Adherent CHOMito-Photina®/H3 Luminescent Protein Cells in FLIPR TETRA System
Introduction
For the discovery and validation of early lead compounds in drug discovery, FLIPRTETR ​​with a light-emitting protein detection application is a simple and reliable high-throughput screening system. The Luminescent Detection Unit includes an Enhanced CCD (ICCD) camera and suspension cell system. The gain-adjustable function of the ICCD camera allows the FLIPR TETRA system to accommodate both dye-based bright fluorescence experiments and chemiluminescent protein experiments with weaker signal strength. This application note describes the experimental results and performance of adherent CHO Mito-Photina®/H3 photoprotein cells in the FLIPRTETRA system.
About the mito-Photina cell line
CHO mito-Photina/H3 is a cell line supplied by Axxam SPA (Milan, Italy) that expresses the mitochondrial target deactivating photoprotein and the G protein coupled receptor histamine 3 (H3), together with the Gαq protein. When incubated with coelenterazine, Apo- Photina is expressed in the cell to form an activated Photina molecule that emits blue light upon binding to the intracellular calcium ion caused by activation of the GPCR. The chemiluminescent detection type ICCD camera can easily detect high correlation light unit (RLU) signals from Photina cells without camera saturation. The use of the camera's gain-adjustable function allows the FLIPR TETRA system to detect very low light signals as well as fewer cells in chemiluminescence experiments, reducing the need for cell culture.
material
Media: Dulbecco's MEM/Nutrients mixed with F-12 and added HEPES (Cat. #11039-047), 1.35 mM sodium pyruvate (Cat. #11360-070), 10% FBS (Cat. #10082-147), 1% penicillin/streptomycin (Cat. #15070-063). 500 μg/mL Geneticin (Cat. #10131-027). 1% L-glutathione (Cat. #25030-081), all from Invitrogen.
Versene (Invitrogen Cat. #15040-066)
PBS without calcium and magnesium ions (Invitrogen Cat. #14190 or similar)
0.05% Trypsin with EDTA (Invitrogen Cat. #25300-054)
Natural coelenterazine (recommended by cell provider)
BSA Media: DMEM/F12 (without phenol red) with 15 mM HEPES (Invitrogen Cat. #11039-021), 0.1% BSA (Fraction V heat shocked, low endotoxin, low protease (Sigma Cat. #A6003 or similar) Water for injection (Irvine Scientific Cat.#9309 or equivalent)
Tyrode Buffer (pH 7.4)—alternate for BSA Media (all chemicals from Sigma): 130 mM NaCl, 5 mM KCl, 2 mM CaCl 2 , 1 mM
384-well black wall bottom recording board (Corning Cat. #3172 or equivalent)
method
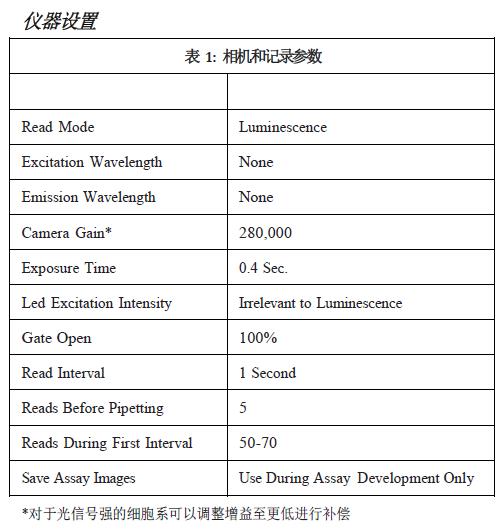
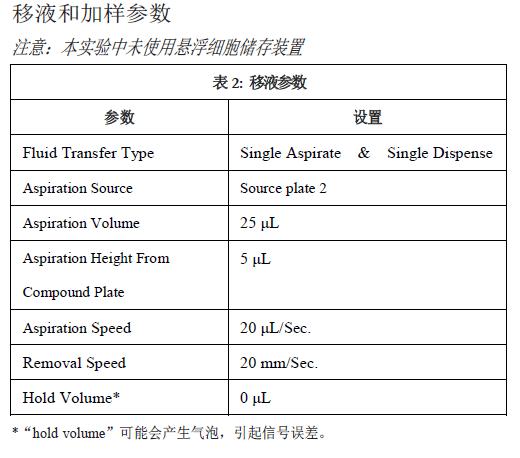
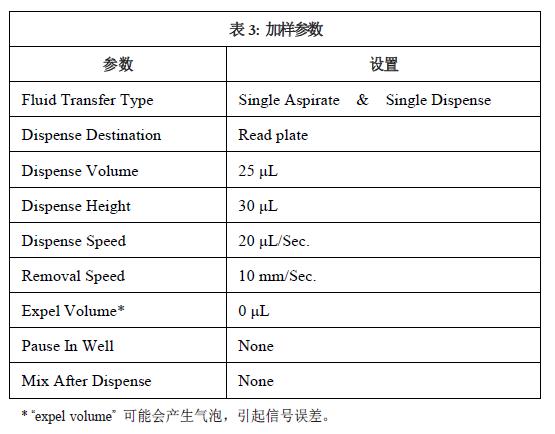
The chemiluminescence experiments of luminescent proteins involve the preparation of cells, compounds and instruments. The instrument settings are described in Tables 1, 2, and 3. For more information, refer to Section 7.13 of the Chemiluminescence Protocol in the FLIPR TETRA System Instruction Manual.
Cell preparation
Step 1: Before the experiment, the cells were resuscitated and cultured under the conditions of screening for antibiotics, following the instructions of the cell provider.
Step 2: Digest the cells from the flask with trypsin one day before starting the experiment
Step 3: Seed the cells on a 384-well black-walled recording plate in an antibiotic-free medium at 2,500-10,000 cells per well. Incubate overnight at 37 ° C and 5% CO 2 .
Step 4: On the morning of the experiment, remove the medium from the wells of the recording plate.
Step 5: Add 25 μL of BSA medium or 5 μM of natural coelenterazine to each well in a low light intensity environment.
Step 6: Cover the plate cover, then wrap the foil to protect it from light, then incubate at 22 ° C or lower for 4-6 hours to optimize the coelenterazine reaction.
Compound plate preparation
The compound plate can contain enough volume to meet the needs of 3-4 recording plates. Prepare a 384-well polypropylene compound plate and reserve enough volume to avoid air bubbles when pipetting the cell plate.
Experimental run
Step 1: Load the 384-well black FLIPRTETRA system gun head
Step 2: Create a FLIPRTETRA system experiment in ScreenWorks® software. See Table 1 for camera and recording parameter settings. Pipetting and loading parameters are shown in Tables 2 and 3. No suspension cell device was used in this experiment. Maintenance and emptying volume settings during pipetting may create bubbles that cause signal errors. Save the file before updating the software settings.
Step 3: Ensure that coelenterazine is added to the cell plate and protected from light. Choose the appropriate method below to complete your experiment.
Here are the experimental scenarios for three different processes:
Only agonist experiments
Step 4: Add 25 μL of 2X agonist to the cell plate and record the data for 30-60 seconds.
or
Agonist and inhibitor experiments
Step 4: Add 25 μL of 2X inhibitor to the cell plate and record the data.
Step 5: Incubate the cell plate in the dark for 15-30 minutes.
Step 6: Add 25 μL of 3X agonist to the cell plate and record the data
or
Pre-experimental inhibitor addition
Step 4: It is acceptable to add the inhibitor to the cell plate and incubate before the experiment.
Step 5: Incubate the cell plate in the dark for 15-30 minutes.
Step 6: Add 25 μL of 3X agonist to the cell plate and record the data.
Data reduction
The data reduction of the RLU signal shown in Figure 1 is calculated in the ScreenWorks software based on the response/background of the signal. The RLU signal simplification shown in Figure 2 is calculated in ScreenWorks software based on the maximum-minimum value of the signal. The dose-response curve was calculated using GraphPad Prism® 3 software.
result
Scope of assessment
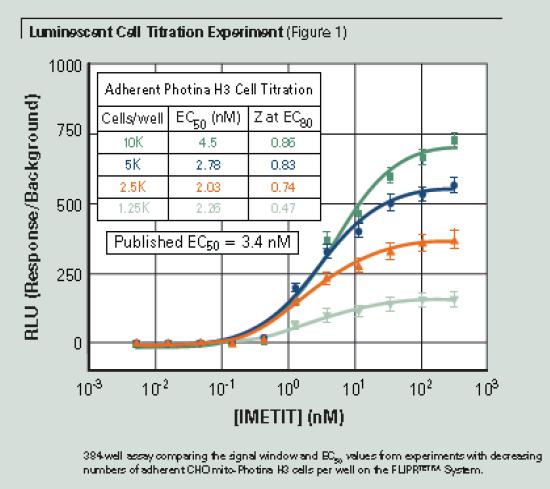
In this experiment, the cell number gradient was used to determine the detection range of the experiment. The cells were incubated overnight at 37 ° C and 5% CO 2 to allow sufficient adherence before the experiment. The FLIPRTETRA system with a chemiluminescent detection component adds an agonist to the adherent cells when recording. The RLU value can be used both for the calculation of the maximum minus the minimum value and for the calculation of the effect relative to the background signal. The calculated EC 50 values per well in experiments with results 1250 cells per well in 000 cells was similar. Figure 1 shows the increase in the signal window as the number of cells increases. At 2500 cells per well, the signal window is approximately 300:1 compared to the FLIPR® Calcium 4 Assay Kit fluorescence signal window of approximately 4:1 (data not shown). Subsequent adherent cell experiments were performed using 2500 cells per well and overnight plate culture. The adherent cell Photina assay can be performed at medium to low cell densities and gives good Z values ​​at EC 80 screening concentrations. The signal effect shown in Figure 1 is calculated according to the reaction/background.
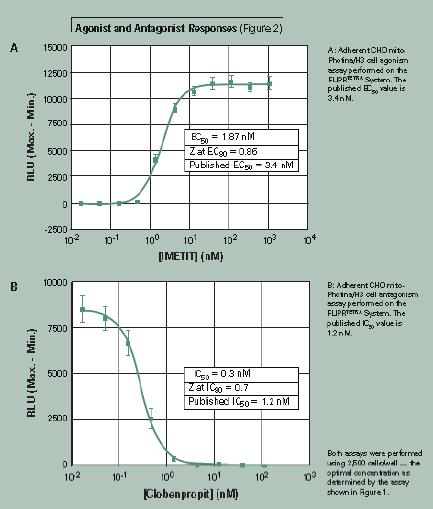
Agonist and inhibitor responses
In the experiment shown in Figure 2, the agonist and inhibitor effects were compared under optimized cell concentration conditions. A 384-well black-walled cell plate was seeded with 2500 CHO mito-Photina/H3 cells per well and cultured overnight at 37 ° C and 5% CO 2 . After the medium was removed, 5 μM of natural coelenterazine was added to the assay buffer and incubated for four hours in the dark.
In this experiment, the data reduction result is calculated by subtracting the minimum value from the maximum value of the recording phase. EC 50 values agonist and inhibitor IC 50 values reported in the literature is the same.
in conclusion
The FLIPR TETRA system with chemiluminescent detection components provides superior signal detection. Not only does a camera detect fluorescence and chemiluminescence, but the camera's gain is adjustable to achieve an unmatched dynamic range for chemiluminescence detection. The system has proven to be very useful in performing high-throughput screening experiments on chemiluminescence. The photoprotein-based calcium flow assay in the FLIPR TETRA system with chemiluminescent detection components has the following advantages:
Ø The system's unparalleled dynamic range detects all light signals from very bright to dim.
Ø Lower background noise, higher signal to noise ratio and larger signal window
Ø Lower cell number and reduce cell culture workload
Ø The experiment is more flexible, and the adherent cells or suspended cells can be tested.
Ø Long-term experiment that can last for more than 6 hours
Ø Data results that are highly consistent with the literature are available for both activation and inhibition experiments.
Ø Eliminate the interference of compound autofluorescence on the results
Ø Lower reagent cost
references
S. Bovolenta, M. Foti, Lohmer, S., S. Corazza. Development of a Ca2+-Activated Photoprotein, Photina, and Its Application to High-Throughput Screening. J Biomol Screen 2007; Vol. 12(5) 694–704 .
China Extract Powder For Use As Dietary Supplement Extract Powder, Extract Powder Manufacturer
Shaanxi Kang New Pharmaceutical co., Ltd. , https://www.bodybuildingoil.com