[Injection container headspace oxygen content process monitoring introduction]
The need to monitor the headspace oxygen content of the injectable container is due to the need to ensure the stability and efficacy of the oxygen sensitive product. In addition to reduced performance and shelf life losses, exposure of such products to oxygen can result in product discoloration, dissolution and appearance changes, and even toxicity, or other pharmacological properties associated with negative effects.
In the development of oxygen-sensitive products, research is needed to investigate the interaction of formula with oxygen. Stability studies at the end of the shelf life confirmed that the product was guaranteed to remain at the specified oxygen concentration. Such studies have made it possible to adapt the technical parameters of the headspace oxygen concentration at the beginning of the primary package. After the zui, the headspace oxygen concentration is often monitored as an in-line process control (IPC) of the purge system during the filling process to bring the headspace oxygen concentration below the required technical requirements.
Unfortunately, existing analytical methods for monitoring headspace oxygen in injection containers are slow and destructive. This makes headspace oxygen detection a lot of time and resources. Traditional destructive techniques, such as electrochemical methods or gas chromatography, are difficult to perform or provide real-time feedback on the filling process online. Measuring the destructive nature also means that these traditional methods cannot be used for 100% inspection of products . Once the septum of the container is pierced for headspace sampling, the integrity of the container is destroyed. The product is therefore damaged and costs are incurred, and because it is destructive, it is also necessary to process the sample for analysis.
The LIGHTHOUSE platform for fast and non-destructive headspace oxygen content analysis can help to simplify the monitoring of the sweeping effect on the filling line. The automatic VISTA headspace oxygen detection machine and benchtop FMS headspace oxygen analyzer enable rapid and non-destructive detection of the oxygen concentration of the sealed injection container. The reliability and ease of operation of the platform can be used for online installation activation of production environments. The fast, non-destructive nature of the measurement allows real-time feedback on the filling process, 100% inspection of the container , without the need to deal with damaged products.
The patented LIGHTHOUSE platform uses high-sensitivity detection technology also known as frequency modulation spectroscopy ( FMS ). Light emitted from the near-infrared semiconductor laser is frequency modulated to match the internal vibration frequency of the oxygen molecules. The headspace oxygen concentration was measured by measuring the absorption of the laser through the headspace of the vessel.
[headspace oxygen content analysis experiment]
The purpose of the experiment is to demonstrate that the FMS rapid non-destructive technique is used for headspace oxygen content analysis and the traditional destruction techniques commonly used by Zui for headspace oxygen detection, while demonstrating that online oxygen monitoring uses an automatic detection model.
Sample groups with different oxygen concentrations were placed in different injection containers, and then the headspace oxygen concentration of these sample groups was tested using a non-destructive LIGHTHOUSE oxygen analyzer. One sample set was then analyzed using a conventional destructive electrochemical method and the second sample set was analyzed using a conventional destructive gas chromatography method. Then determine the correlation between LIGHTHOUSE FMS technology and destructive methods.
For the online experiment using the automatic headspace oxygen detection platform, two experimental settings were used, and the * settings involved the use of an automatic headspace oxygen platform directly after filling the oxygen sensitive product. Then change the purge rate of the N2 purge system when the vial is filled. The effect of changing the headspace oxygen concentration is then monitored in real time. The second online setup involves the production of headspace oxygen standards using certified gas mixtures. The certified oxygen vial standard material is then continuously run through an automated headspace oxygen platform to generate statistical data to confirm the accuracy and precision of the online oxygen measurement.

[The result of headspace oxygen content analysis]
* The analysis results of one sample group are shown in Figure 1 . The liquid product sample was filled in a 10 mL ampoule and produced a series of headspace oxygen concentrations by varying the purge rate on the filling line. The table lists the average oxygen concentration from 10 lossless LIGHTHOUSE measurements, as well as the standard deviation and relative standard deviation of the 10 measurements. The LIGHTHOUSE results are then compared to a destructive measurement of the electrochemical method. The comparison shows a good correlation between these two measurement techniques. Unfortunately, it is impossible to determine the accuracy of electrochemical techniques. Because the sample was destroyed by measurement. Similar correlation experiments were also performed using gas chromatography as a destructive method.

* The results of an online experiment are shown in Figure 2 . The bottle of Zui was initially charged N2 and the purge was closed. The figure shows that the oxygen concentration of the * vials is the same as the atmospheric oxygen concentration.
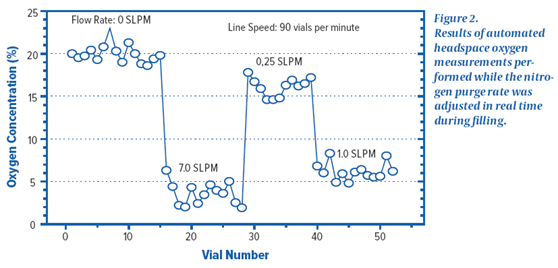
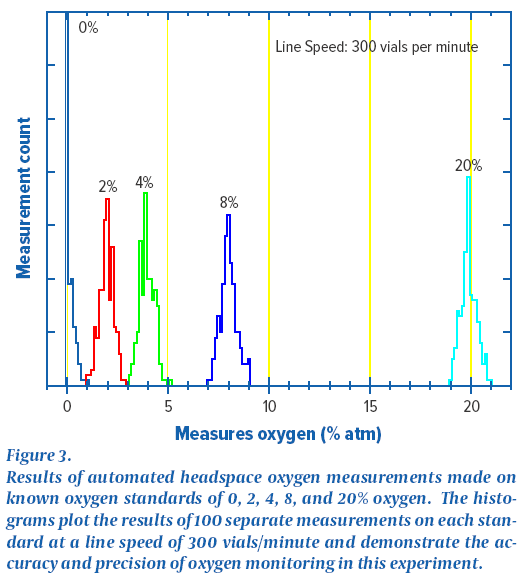
When the vial is filled, the purge rate is then changed in real time. Increasing the purge rate to 7.0 standard liters per minute ( SLPM ) resulted in a headspace oxygen concentration of approximately 3% for the vials . Further changes in the purge rate confirm the relationship between the headspace oxygen concentration change and the purge rate. The filling in this demonstration was done at a line rate of 90 bottles per minute and a real-time feedback of the headspace oxygen concentration in each vial was obtained using an on-line headspace oxygen system. The results of the second online experiment are shown in Figure 3 . The figure depicts that online measurements are made with a set of certified oxygen standards. This set of standards was continuously circulated through an automatic headspace oxygen measurement system at a rate of 300 bottles per minute so that each standard was measured 100 times. The histogram in Fig. 3 is a plot in which each of the reference materials was measured 100 times. The histogram results give a complete graph of the machine's performance. The peak value of the distribution is related to the measurement accuracy. The width of the distribution is related to the precision between measurements. The results in Figure 3 show that at a line speed of 300 bottles per minute, the platform can resolve headspace oxygen concentrations between 0% and 2% . Further experiments have shown that better sensitivity can be achieved by lowering the line rate.
[Conclusion of headspace oxygen content analysis]
The LIGHTHOUSE platform is capable of measuring the headspace oxygen concentration in a sealed injection container in a fast and non-destructive manner. Comparative experiments show that LIGHTHOUSE technology is highly correlated with traditional destructive technology for on-line process control of headspace oxygen concentration during the filling process. Automated machines have been adopted, validated and registered by regulatory authorities for indoor online headspace oxygen monitoring worldwide. Its fast and non-destructive advantages of headspace oxygen content analysis include the following:
- Quick feedback on the purge effect during the filling process
- Expandable to 100% inspection of products , automatic process monitoring ensures quality
- Measurement has nothing to do with operator expertise
- Efficient and accurate optimization and verification of the N2 purge system
- No waste in dealing with damaged products
- Low continuous supplies cost
We're Professional Supplier Extract Powder manufacturers and suppliers in China specialized in providing high-quality products at low price. We warmly welcome you to buy or wholesale bulk Supplier Extract Powder for sale here from our factory. For a free sample, contact us now.
Supplier Extract Powder,Supplier Extract ,Supplier Powder Manufacturer in China
Shaanxi Kang New Pharmaceutical co., Ltd. , https://www.kangnewpharmas.com