Boob tape, breast lift tape
Boob tape is based on 95% cotton and 5% Elastic Spandex fabric. The material is safe, soft, breathable, which makes you comfortable in it. It has super strong elasticity, lateral stretching is more than 230%. It is coated with imported wave pattern glue, which ensures the tape has stable adhesion and easy to remove, do not hurt your skin. It has a certain waterproof effect, the adhesion will not be reduced even you are sweaty or swimming. It has wonderful lifting and gathering effect, let you have a proud chest curve.

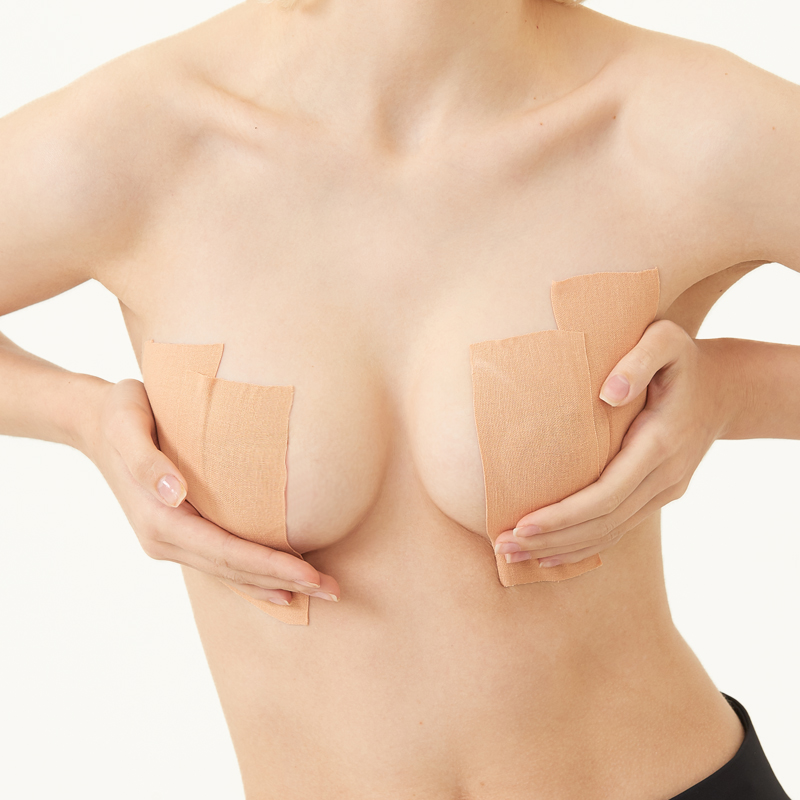
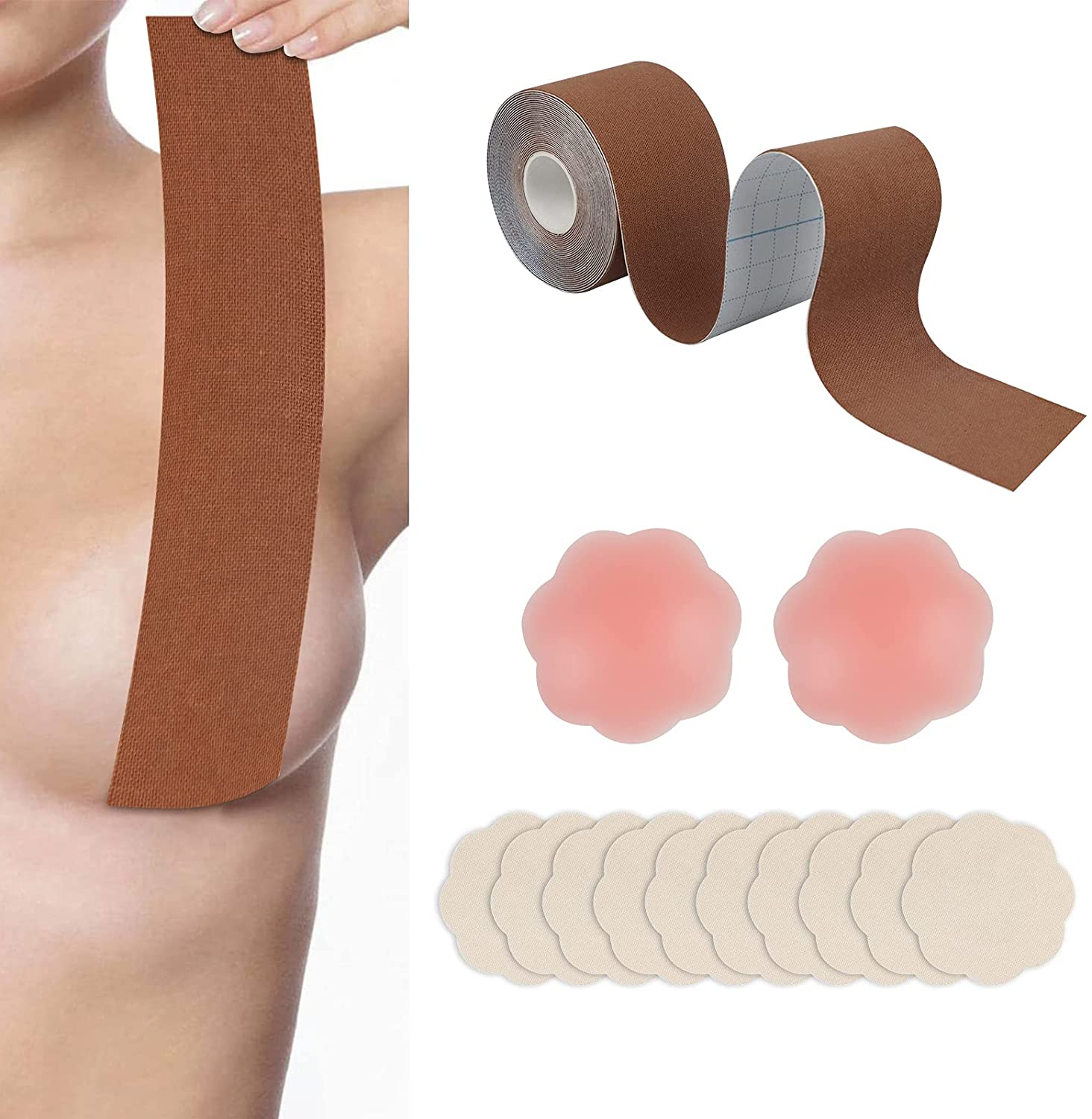
boob tape,breast lift tape,boob tape and nipple cover,boob lift tape,breast tape
Kunshan Jieyudeng Intelligent Technology Co., Ltd. , https://www.jerrytapes.com