Release date: 2018-03-15
The World Health Organization (WHO) predicts that neurodegenerative diseases will replace cancer as the second most deadly disease in humans in 2040. There are no effective treatments for these diseases so far, so it is particularly urgent to understand the pathogenesis of these diseases. Many neurodegenerative diseases, including Alzheimer's disease, Parkinson's disease, Huntington's disease, and amyotrophic lateral sclerosis (ALS), have a common set of characteristics. It is the protein aggregation caused by the environmental stress caused by genetic mutation and individual aging. Based on this, there are two hypotheses about the mechanism of disease occurrence: the gain of toxic function brought about by protein aggregates and the loss of critical physiological function caused by gene mutations ( Winklhofer et al., 2008).
Recently, Professor Baumeister from the Max Planck Institute for Biochemistry in Germany collaborated with Prof. Edbauer from the German Center for Neurodegenerative Diseases and Prof. Kochanek from Ulm University. The work completed by Dr. Guo Qiang was successively completed in the journal Cell and Nature within one month. Published an article entitled "In Situ Structure of Neuronal C9orf72 Poly-GA Aggregates Reveals Proteasome Recruitment" and "The cryo-electron microscopy structure of huntingtin", using frozen electron tomography and single-particle three-dimensional reconstruction techniques to separate cells The structure of in situ protein aggregates and their microenvironment and the structure of human huntingtin proteins provide new ideas for understanding the pathogenesis of neurodegenerative diseases.
first time
A research paper entitled "In Situ Structure of Neuronal C9orf72 Poly-GA Aggregates Reveals Proteasome Recruitment" published in Cell on February 8 (Guo et al., 2018a), which resolves a repeat in situ in rat nerve cells. The structure of polypeptides (poly-GA) aggregates provides favorable support for the "acquired toxicity mechanism" hypothesis in disease development.
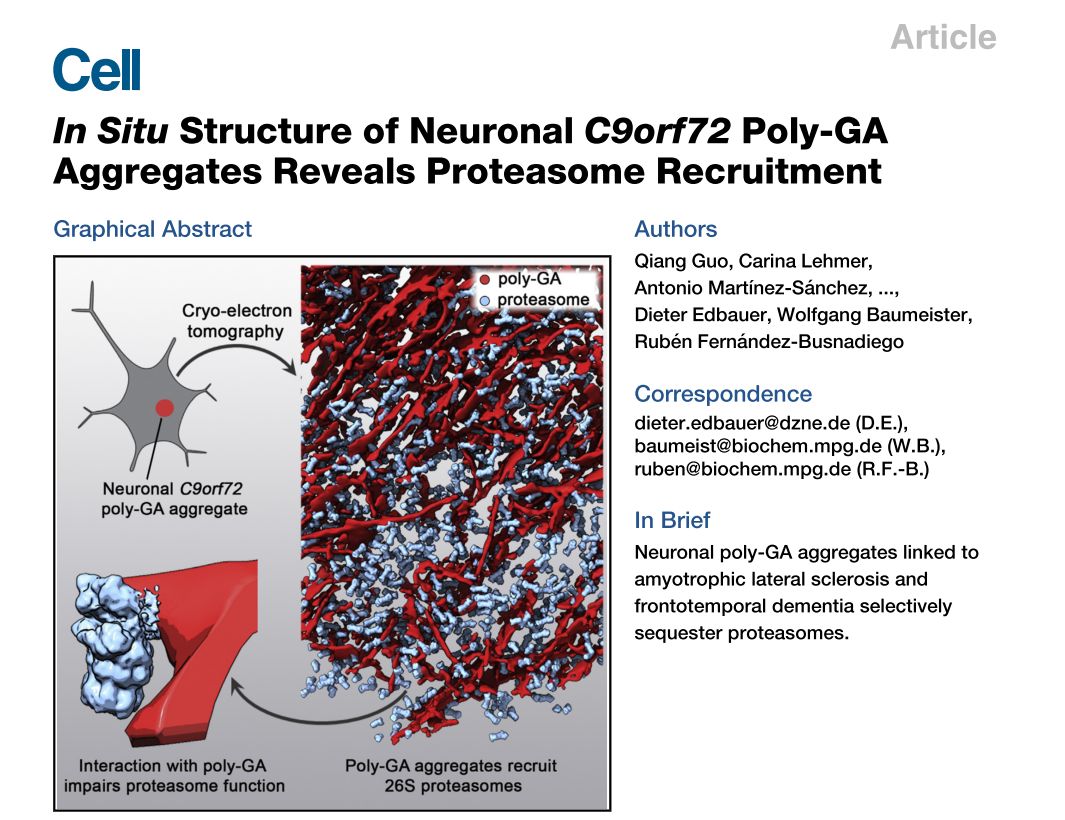
The study suggests that multicopy mutations in the C9orf72 gene are the most important genetic factors leading to amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). The multicopy sequence is translated under pathology into five different repeating short peptides, which in turn form protein aggregation. In this work, one of the most common repeating short peptides (poly-GA) was selected as the research object, and the structure of the protein aggregation was analyzed in situ in rat nerve cells. The researchers found that unlike the spiral fiber-like structures formed by proteins such as Tau, A-beta, Huntingtin and other proteins associated with neurodegenerative diseases, the protein formed a ribbon-like structure with varying lengths in the cells.
In addition, the protein aggregates recruit a large number of proteasomes, resulting in a proteasome concentration of up to 30 times the normal concentration in the protein aggregate region, while other biomacromolecules such as ribosomes and chaperones are excluded from the aggregate. Further cryo-electron microstructural analysis showed that, although the concentration increased, contact with protein aggregates resulted in a large number of proteasomes being inactivated. As a result, the amount of proteasome expression of the cells at the overall level did not change, and the number of functional proteasomes was greatly reduced, which may be the cause of cytotoxicity caused by protein aggregation.
It is worth mentioning that, at the level of cryo-electron microscopy technology, the multi-scale sample tracking imaging of the electron density map from the microscopic level of the optical microscope to the resolution of about one nanometer is realized for the first time by using various imaging techniques.
Second
A research paper entitled "The cryo-electron microscopy structure of huntingtin" (Guo et al., 2018b), published in Nature on March 1st, reports the cryo-electron microscopy structure of human huntingtin protein, which helps humans Learn about the physiological functions of huntingtin, a key protein in Huntington's disease.

Huntington's disease is an autosomal dominant neurodegenerative disease caused by mutations in the huntingtin gene. Although the disease-causing gene of the disease was identified 25 years ago, the structure of the protein and its exact function are still unknown. In this study, the researchers found that the huntingtin-binding protein HAP40 stabilizes the three domains of Huntingtin, which makes the resolution of high-resolution structures possible. Finally, the researchers first obtained the atomic resolution structure of the protein.
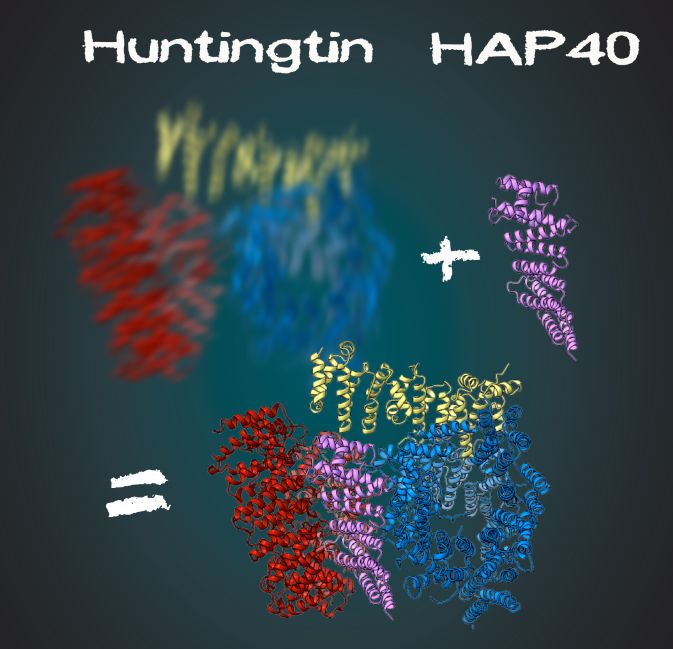
Huntingtin-HAP40 complex structure (photo: Gabriele Stautener, ARTIFOX)
The current most promising approach to the treatment of Huntington's disease is to use the silencing of the Huntingtin gene, which targets the mRNA of the gene by antisense polynucleotides, and then by gene degradation to achieve gene silencing. However, the drawback of this method is the inability to distinguish between the mutant huntingtin gene and the normal huntingtin gene. Therefore, further understanding of the function of huntingtin protein has important and far-reaching significance for optimizing this treatment plan or developing new treatment options.
Dr. Guo Qiang is the co-first author of the two papers of Cell and Nature. These two work have also been reported and paid attention by A LZFORUM, ScienceDaily and Max Planck Society. Teepu Siddique, a neurobiologist from Northwestern University in Chicago, believes that this work on amyotrophic lateral sclerosis (ALS) is very important because it has not received enough attention in the past. Yun-Ru (Ruby) Chen, a colleague from the “Academia Sinica†in Taipei, said that the above research will have a major impact in related fields.
References and materials:
1. Guo, Q., Lehmer, C., MartÃnez-Sánchez, A., Rudack, T., Beck, F., Hartmann, H., Pérez-Berlanga, M., Frottin, F., Hipp, MS, Hartl, FU, et al. (2018a). In Situ Structure of Neuronal C9orf72 Poly-GA Aggregates Reveals Proteasome Recruitment. Cell 172, 696–705.e12.
2. Guo, Q., Bin Huang, Cheng, J., Seefelder, M., Engler, T., Pfeifer, G., Oeckl, P., Otto, M., Moser, F., Maurer, M., Et al. (2018b). The cryo-electron microscopy structure of huntingtin. Nature 555, 117–120.
3. Winklhofer, KF, Tatzelt, J., and Haass, C. (2008). The two faces of protein misfolding: gain- and loss-of-function in neurodegenerative diseases. EMBO J. 27, 336–349.
4. https://
Source: BioArt
Canned Fish,Fish Tin,Canned Sardines Tin,Fish Canned
ZHANGZHOU TAN CO. LTD. , https://www.zztancan.com