Introduction:
Establishing an in vitro model that physiologically mimics the in vivo environment is critical for understanding neurological disease mechanisms and drug discovery. Stem cell-induced neuronal cell culture has great potential for compound screening and disease model establishment in a 3D environment. 3D culture is considered to be the closest in vitro culture method to human tissue, and it is a good simulation of the in vivo environment, regardless of structure, cell growth characteristics, and interaction between cells and cells and cells and matrix. This presentation describes a new approach to neurodegenerative disease and neurotoxicity screening using iPSC-induced neuronal cells for neuronal biopsy at the 3D level on the OrganoPlate® high-throughput microfluidic platform. OrganoPlate® is a combination High-throughput screening platform with the latest 3D culture technology and microfluidic technology, based on 96-well tissue chip, can perform long-term live cell detection, meet screening needs, and is compatible with common laboratory equipment and automation systems, such as ImageXpress® MicroConfocal Connotation imaging and analysis system.
material:
• OrganoPlate® board (MIMETAS)
• Human iPSC-induced neuronal cells iCell® Neurons (Cellular Dynamics International)
• Neural Cell Culture Medium (Cellular Dynamics International)
• Matrigel (Corning)
• Calcein AM (Life Technologies)
• MitoTracker Orange (Life Technologies)
• Hoechst (Life Technologies)
• Saponin (Sigma)
• PBS (Sigma)
• Αντι-β-tubulin III (TUJ-1) Antibody (BDBiosciences)
• ImageXpress Micro Confocal Confocal High Content Imaging and Analysis System (MolecularDevices)
• MetaXpress High Content Imaging and Analysis Software 6.2 (Molecular Devices)
Form a neural network in 3D glue:
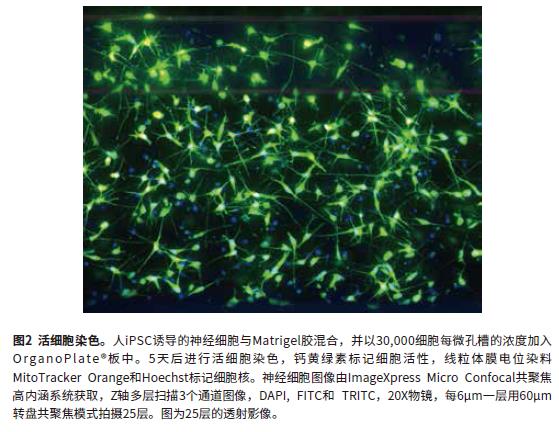
The cell culture, compound treatment, and cell staining steps are described in terms of microfluidic procedures. Confocal imaging mode was used to evaluate the phenotypic changes and cell viability of nerve cells in 3D gel. Human iPSC-induced nerve cells were mixed with Matrigel gel at a final concentration of 7 mg/mL and added to the OrganoPlate® plate at a concentration of 30,000 cells per microwell. At the time of seeding, the suspension of glue and cells should be placed on ice to prevent the Matrigel gel from solidifying. The volume added to a microfluidic channel was adjusted to be between 1-1.4 μL depending on the concentration of the gel and the concentration of the cells. After seeding, the microfluidic plate was placed in a 37 ° C 5% CO 2 incubator for 30 minutes to solidify the gel. Then, 50 μL of the medium was added to the inlet and outlet of the microfluidic channel, respectively, and placed in an incubator. Synapses are formed after 24 hours and can be cultured for up to 14 days. To evaluate the feasibility of the OrganoPlate microfluidic plate to study neurotoxicity and neuronal development, several known synaptic inhibitors were selected to treat neurons. The cells were added to the compound 24 hours after the culture, the culture was continued for 5 days, and the medium containing the compound was changed every two days, and the compound was added to the microfluidic well with a suitable gradient. The formation of the neural network is monitored in real time by transmitted light. A mixture of three living cell dyes is added to the cells for evaluation of nerve cell viability: live cell dye calcein (1 μM), mitochondrial membrane potential stained MitoTracker Orange (1 μM), and nuclei Dye Hoechst (1 μM). The mixed dye was added to the microfluidic chip wells and incubated for 60 minutes and then washed away with medium. Alternatively, the cells were fixed with 4% paraformaldehyde, 0.01% saponin was added for cell membrane permeabilization, stained with a 1:100 dilution of the neuronal marker β-tubulin III (TUJ-1) primary antibody and fluorescent secondary antibody, stained with Hoechst. Nuclei. Primary antibody incubation requires 4 ° C overnight.


Phenotypic analysis of 3D culture:
High content imaging and analysis systems were used to evaluate the effects of compounds on neural networks. To better study neural cell viability and synaptic growth in 3D gels, we optimized confocal imaging modalities and analytical methods. Neuron images were acquired by the ImageXpress Micro Confocal confocal high-content system using 10x, 20x, and 40x objectives and a Z-axis sequence above and below the focal plane (Figure 1). Images of 17-30 layers with a layer spacing of 3-10 μm were taken and contained approximately 150-300 μm thickness. All images were saved and used for further 3D analysis and 2D transmission analysis (Maximum Transmission or BestFocus).
Phenotypic analysis of 3D culture:
High content imaging and analysis systems were used to evaluate the effects of compounds on neural networks. To better study neural cell viability and synaptic growth in 3D gels, we optimized confocal imaging modalities and analytical methods. Neuron images were acquired by the ImageXpress Micro Confocal confocal high-content system using 10x, 20x, and 40x objectives and a Z-axis sequence above and below the focal plane (Figure 1). Images of 17-30 layers with a layer spacing of 3-10 μm were taken and contained approximately 150-300 μm thickness. All images were saved and used for further 3D analysis and 2D transmission analysis (Maximum Transmission or BestFocus).
Maximum transmission 2D image analysis:
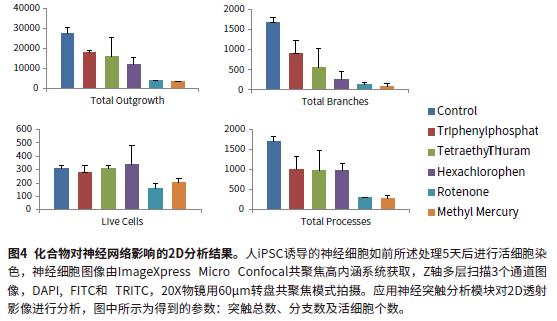
Image analysis using MetaXpress® high-content image acquisition and analysis software. Fully automated two methods for quantitative analysis of images: 2D analysis after transmission and 3D level analysis of Z-stack. For 2D transmission image analysis, the Z-stack sequence image was subjected to Maximum transmission and then analyzed using a software-prepared synaptic analysis module. Output parameters for evaluating cell phenotype and neural network complexity at different time points include: total synaptic length, number of synapses, cell number, and cell viability. The transmitted light image was analyzed by 2D transmission using the Best Focus algorithm. 2D images are fast and can accurately assess the extent of neural networks. However, 2D analysis has certain limitations. This method cannot calculate parameters such as volume, and only results in a single layer. Figures 2 and 3 show the fluorescence synthesis maps, and the mask images of the control and rotenone treatment groups were analyzed by the synapse module. Analytical parameters include: total length of synapses, number of synapses, and total number of cells (cell bodies). Figure 4 shows that the synaptic length, number of synapses, and cell viability are reduced with compound treatment. Neurotoxic compounds include: triphenyl phosphate, tetraethyl thiuram, hexachlorophenol, rotenone, and methylmercury. (all 10μM).

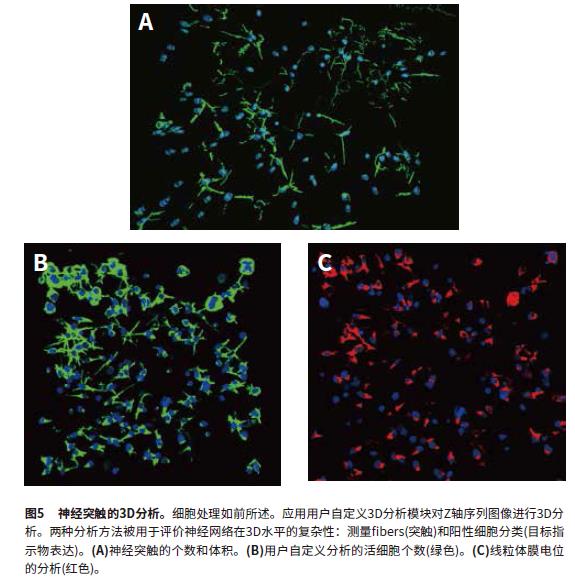
3D visualization and 3D image analysis:
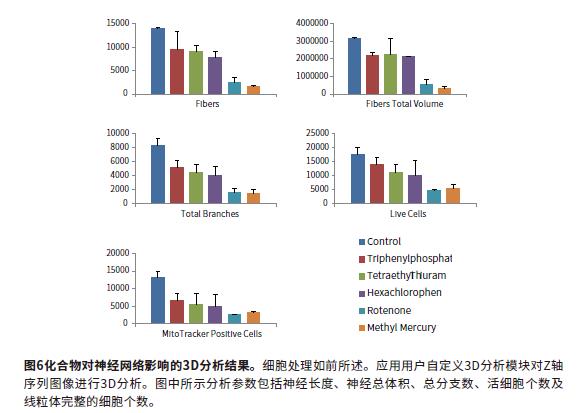
MetaXpress software can reconstruct the Z-axis sequence image into a 3-dimensional stereo structure to observe the nuclear and cellular networks, and perform 3D level analysis. The image uses the "findfibers" function in the user-defined 3D analysis, and the "fibers" value evaluates the synapses; the number of cells is also calculated. The calculation objects are first identified in each layer and then connected in 3D space using the "connect bybest match" function. 3D analysis makes synaptic calculations more accurate and is a better way to evaluate neural connections. The parameters obtained from the 3D analysis include the number of synapses, the cell volume, the total synaptic volume, the number of branches, and the number of nodes. The total number of cells is identified by the "sphericalbjects" in the Hoechst staining channel. Figure 5 shows the mask that identifies the "fibers" and "nuclei". Another user-defined module analyzes cell viability characteristics (calcein-positive cell bodies) and mitochondrial integrity (MitoTracker Orange-positive cell bodies). Cell sorting analysis can obtain the number of viable cells (calcitoxin-positive cell bodies) and the number of cells with intact mitochondria (MitoTracker Orange-positive). The calculation results include the number of cells (total nuclei), the number of living cells (Calcein AM positive), and the cell volume and fluorescence intensity. Figures 5B and 5C show the recognition masks for "Calcein AMpositive cytoplasm", "MitoTracker positive cytoplasm" and "nuclei". Figure 6 shows several characteristic parameters of a compound affecting a neural network: the number of synapses, the number of nodes, and the number of viable cells and the number of intact mitochondria.
3D analysis of neurotoxicity using OrganoPlates:
Phenotypic analysis involves quantitative 3D analysis of neural networks in terms of degree and complexity, resulting in multiple parameters. In this neural model system, we evaluated the model's repeatability, multiple parameters, and tested several known neurotoxic compounds. Through these analytical methods, we obtained accurate concentration-dependent inhibition curves of compounds on complex neural networks. It was therefore verified that this model can be used for high-throughput multi-parameter prediction of neurotoxicity of compounds.
in conclusion:
We have established a quantifiable confocal high-content imaging method that combines the use of MIMETA SOrganoPlates to perform high-throughput 3D phenotypic analysis of the effects of compounds on neuronal viability and shape. Confocal imaging and multiparametric 3D analysis modules provide a means to calculate and count the number of neurons and synaptic features at the 3D level. The 3D analysis results calculate the EC50 value of the compound's effect on nerve cells and help screen for toxic compounds.
references:
1. Chang, TT; Hughes-Fulford, M (2008). Monolayer and Spheroid Culture of Human Liver Hepatocellular Carcinoma Cell Line Cells Demonstrate Distinct GlobalGene Expression Patterns and Functional Phenotypes.Tissue Eng Part A. 15(3): 559-567
2. Kunz-Schughart, LA et al. (2004). The use of 3-D cultures for high-throughput screening: themulticellular spheroidmodel. J Biomol.Screen. 9(4): 273-285
3. Trietsch, SJ et al. (2013). Microfluidic titer plate forstratified 3D cell culture.Lab Chip. 13: 3548-3554
4. VanDuinen, V et al. (2015). Microfluidic 3D cell culture: from tools to tissue models. Curr.Opin.Biotechnol. 35:118-26
5. Sirenko, O et al. (2014). High-Content High-ThroughputAssays for Characterizing the Viability and Morphology of Human iPSC-Derived Neuronal Cultures. Assay and DrugDev. Technologies.12(9-10): 536-47
6. Vulto, P et al. (2011). Phaseguides: a paradigm shift inmicrofluidic priming and emptying. Lab Chip. 11:
1596-1602
7. Wevers, NR et al. (2016). High-throughput compoundevaluation on 3D networks of neurons and glia in amicrofluidic platform. Sci. Rep. 6: 38856
China Natural Plant Herbal Extract,Lotus Leaf Extract,Curcuma Extract,Grape Seed Extract, we offered that you can trust. Welcome to do business with us.
Our healthway supply Medical Intermediate Herbal Extracts Natural Plant Herbal Extract Super Food Ingredients Fruit Powder Fruit Juice Powder Freeze-dried Powder Vegetable Powder Food Additives Cosmestic Ingredienrs
Icariin NMN powder Cissus Irish sea moss Quercetin Barley grasss, We had passed FDA certificate and ISO 9001 .
Natural Plant Herbal Extract,Lotus Leaf Extract,Curcuma Extract,Grape Seed Extract
Xi'an Healthway Biotech Co.,Ltd , https://www.xianhealthway.com