Release date: 2018-03-08
Recently, Prometic Life Sciences, based in Quebec, Canada, announced that its Inter-Alpha-Inhibitor-Proteins (IAIP) for necrotizing enterocolitis (NEC) has received FDA certification for pediatric rare diseases (Rare). Pediatric Disease Designation). The FDA Pediatric Rare Diseases Certification is designed to help pharmaceutical companies develop innovative therapies for patients under the age of 18 who will be given a redemption voucher for the owner of this rare disease therapy, which will be available for future drug listing applications. Redeem into a priority review. At the same time, the FDA has also granted Orphan Drug Designation to its IAIP program. If the drug is approved, the orphan drug qualification will give it many benefits, including additional regulatory support and a period of market patents.

NEC is one of the most common gastrointestinal diseases in preterm neonates and one of the leading causes of death in neonatal intensive care units. This inflammatory bowel disease, which mainly affects premature infants, can damage the intestinal wall, causing perforation of the intestines, allowing feces to spill into the baby's abdomen, which can cause strong infections and even death. It has been reported that 11% of the very low birth weight babies born before 29 weeks of age are affected by the disease. According to Prometic, the infant mortality rate caused by the disease is as high as 15% to 30%. In addition to the disease-related mortality, the economic costs it imposes on hospitals are also high. According to Prometic, NEC accounts for about 19% of neonatal-related expenditures, and in the United States alone generates about $5 billion in hospitalization costs. Even if surgery can be avoided, the average hospitalization cost for children is as high as $73,000, and hospital stays exceed 22 days for other premature infants. If surgery is required, the average cost is close to $186,000, and the child stays in the hospital for 60 days longer than other premature babies. This is a heavy burden on the child and his family. Therefore, the group still has urgent medical needs to be met.
Prometic's IAIP is a serine protease inhibitor that regulates endogenous protease activity. According to the company, IAIP regulates the body's response to various inflammatory stimuli that include toxins, infectious organisms, tissue and organ damage, etc., and control excessive inflammatory responses. Sepsis refers to systemic inflammation caused by infection. Severe sepsis can lead to a decrease in IAIP and a decrease in the inhibition of protease activity.
Prometic claims that IAIP therapy shows protection in many preclinical sepsis models. In announcing the news, Prometic presented a "gold standard animal model that mimics NEC in humans", showing that the survival rate of the subjects was significantly improved by injecting IAIP into experimental animals.
The FDA's pediatric rare disease certification this time is the company's second pediatric therapy certification from the regulatory body, issued to the IAIP from the company's plasma purification platform. Prometic's plasma purification platform is based on its unique plasma separation technology and is used primarily to develop plasma-derived protein products for the treatment of a variety of rare diseases.
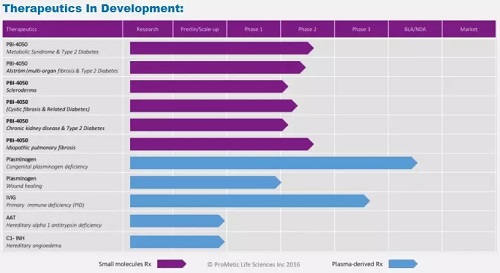
â–²Prometic's R&D pipeline overview (Source: Prometic official website)
Mr. Pierre Laurin, CEO of Prometic, said: "The combination of pediatric rare disease certification and orphan drug qualifications provides us with valuable incentives for us to continue to expand our orphan drug pipeline."
We congratulate Prometic Life Sciences for its clinical benefits in preterm infants.
Reference materials:
[1] Prometic Snags Rare Pediatric Disease Designation from the FDA for its Inter-Alpha-Inhibitor-Proteins
[2] Prometic official website
Source: WuXi PharmaTech
Anesthesia Medical Co., Ltd. , https://www.jssinoanesthesia.com