Last month, the Zhou Bin Research Group of the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences published a new research report on Scientific Reports entitled " Genetic targeting of Purkinje fibres by Sema3a-CreERT2 ", which provides a new type of inheritance. The tool - Sema3a-CreERT2 tool mouse - is used to study the molecular mechanism regulating the function of Purkinje fiber.
In this study, the Sema3a-CreERT2 tool mouse was constructed from Shanghai Southern model organism .
Research Background:
The cardiac conduction system is composed of cardiomyocytes located in the heart wall and highly differentiated by unique functions. Its function is to generate electrical impulses and conduct them to various parts of the heart, so that the atrial and ventricular muscles contract at a certain rhythm. Cardiac contraction begins with the right atrial sinus node (SA knot). The electrical activity is then delayed at the atrioventricular node (AV node) and then rapidly transmitted through the atrioventricular bundle (His beam and bundle) and rapidly spread throughout the ventricular wall. Abnormal function of the heart conduction system can lead to arrhythmias.
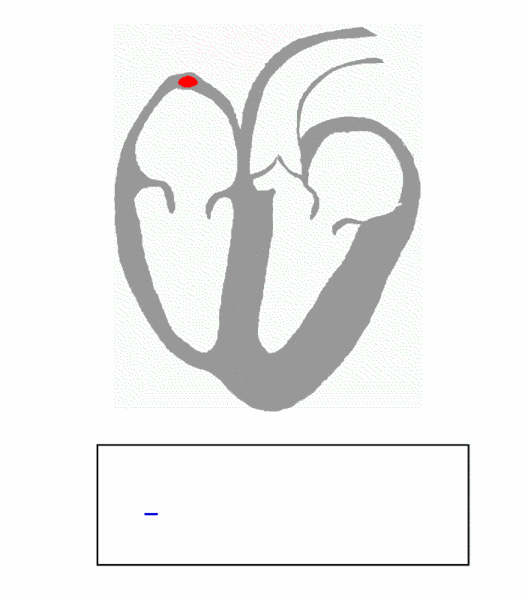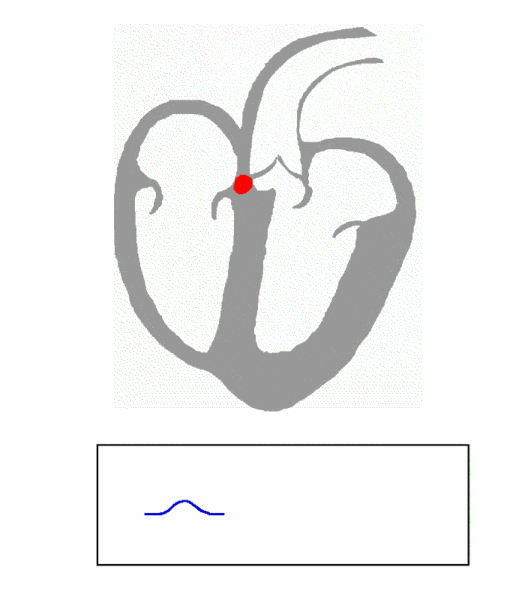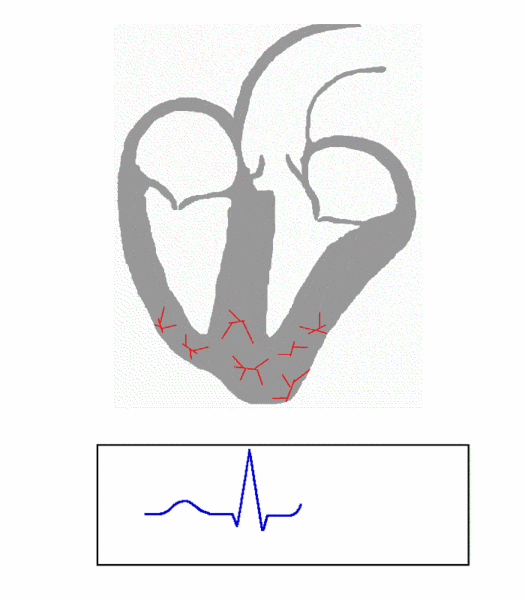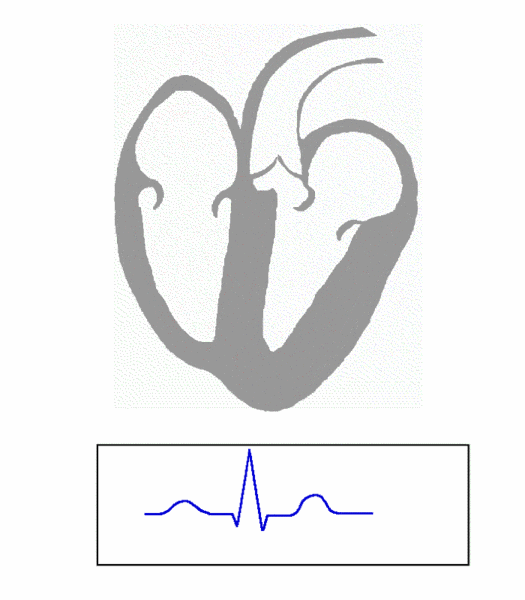
(Source: https://en.wikipedia.org/wiki/file:ECG_Principle_fast.gif)
The protagonist of this article, Purkinje fiber, is the main cell that forms the bundle of the chamber and its branches, also known as the bundle cell. Purkinje fiber intercellular connections and their ion channel mechanisms are the basis for ensuring the normal conduction of cardiac electrical impulses. When intercellular connections and transmembrane ion currents change, the electrophysiology of the Purkinje fiber system changes, leading to arrhythmias. Therefore, understanding the molecular mechanisms that regulate the development, formation, and maintenance of Purkinje fiber will provide explanations and evidence for the pathophysiological processes of arrhythmia.
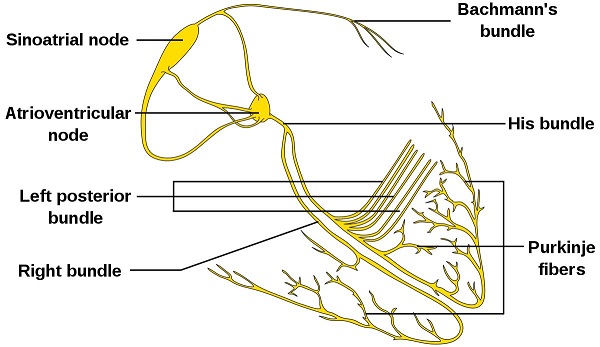
Figure 2. Isolated heart conduction system showing Purkinje fibers
In previous studies, some Cre or CreERT2 tool mice were used to label the cardiac conduction system. such as:
- Hcn4-CreERT2 was used to label the conduction system, including the sinus node, part of the atrioventricular node, His bundle, bundle branch, and Purkinje fiber. However, in addition to the conduction system, Hcn4-CreERT2 also marks part of the endothelial cells of the developing heart.
- Cntn2-Cre is another genetic tool of the cardiac conduction system that specifically targets SA, AV, left and right bundles, and Purkinje fibers.
- Cx40-CreERT2 targets atrial and VCS cardiomyocytes, as well as coronary endothelial cells.
Unfortunately, the lack of specific tools that specifically target Purkinje fibers (without targeting other cells) is not sufficient for the specialized study of Purkinje fibers.
Mouse model:
Sema3a-CreERT2 mice:
Since Sema3a knockout homozygous mice are known to have severe phenotypes of sensory and sympathetic neuron abnormalities, abnormalities in embryonic bone and cartilage, cardiac defects, and postnatal death, inducible CreERT2 is induced using classical ES cell targeting techniques. The Sema3a-CreERT2 mouse model was constructed by inserting into the start codon position of the mouse Sema3a gene. This line was constructed by Shanghai Southern Model organism.
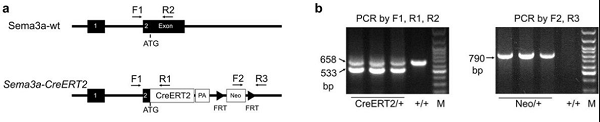
Figure 3. Sema3a-CreERT2 construction strategy for genotypic identification results.
R26-tdTomato mouse: a conditional expression of the tdTomato reporter gene was inserted at the site of mouse Rosa26.
Research result:
A Sema3a-CreERT2 mouse model was constructed.
Sema3a is a member of the pheromone family that is conserved from invertebrates to vertebrates. Studies have shown that Sema3a plays an important role in the development of the nervous system. Sema3a may be a chemical inhibitor that inhibits the growth of axons, especially peripheral axons, thereby maintaining normal neuronal morphological patterns. Overexpression of Sema3a leads to neurological diseases such as schizophrenia; whereas Sema3a deficiency leads to abnormal neuronal systems. In addition, Sema3a also plays a physiological role in immune regulation and cancer development. A recent study showed that Sema3a can maintain normal heart rhythm by regulating the pattern of sympathetic innervation. Although studies have found that Sema3a is expressed in the subgroup of Purkinje fibers and trabecular myocytes, the pedigree tracking of Sema3a in the cardiac conduction system has not been reported so far.
The Sema3a-CreERT2 mouse model was constructed by inserting the inducible CreERT2 into the start codon of the mouse Sema3a gene using classical ES cell targeting technology. Estrogen receptor (ESR) antibody staining was used as a surrogate for Sema3a to detect expression of Sema3a in Sema3a-CreERT2 mouse tissues. ESR (instead of Sema3a) and TNNI3 (cardiomyocyte Marker) were co-stained in E12.5, E14.5 and E19.5 embryonic heart sections. Immunostaining results showed that ESR/Sema3a was mainly expressed in trabecular myocardium, but a small amount of expression was also detected in the embryonic heart dense layer of E12.5 and E14.5 days. On day E19.5, ESR/Sema3a was expressed in subendocardial trabecular myocytes from the ventricular side of the ventricle. ESR/Sema3a was not detected in cardiomyocytes or coronary arteries of dense myocardium. Taken together, these data are consistent with previous studies of Sema3a expression, demonstrating the successful construction of Sema3a-CreERT2 mice.
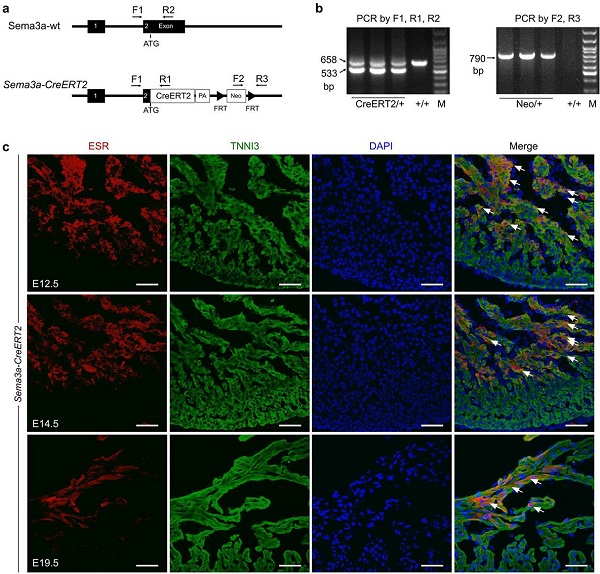
Figure 4. (ab) Sema3a-CreERT2 construction strategy for genotypic identification results. (c) Immunofluorescence staining of Sema3a-CreERT2 mouse heart estrogen receptor (ESR) on E12.5, E14.5 and E19.5 days, indicating Sema3a expression. Arrows indicate Sema3a+ cardiomyocytes.
Sema3a-CreERT2 marks Purkinje fibers in the adult mouse heart.
According to the ESR staining analysis of Sema3a-CreERT2 mice, Sema3a is mainly expressed in trabecular myocytes, rather than in the dense layer. Since Purkinje fibers are mainly concentrated in the innermost layer of the ventricular wall, it is then necessary to determine whether Sema3a-CreERT2 can specifically target Purkinje fibers in the adult mouse heart. Sema3a-CreERT2; R26-tdTomato double positive mice were obtained by mating with R26-tdTomato mice by Sema3a-CreERT2. Only in the presence of tamoxifen, CreERT2 undergoes Cre-loxP recombination from cytoplasmic translocation to the nucleus, which permanently marks tdTomato red fluorescence (RFP) in Sema3a+ cells and their progeny (Fig. 5b).
Tamoxifen induction was given to 8-12 week old mice, and cardiac tissue samples were collected 48 hours after tamoxifen treatment, and sparse RFP+ cells were found in atrial myocytes (Fig. 5c-d). To determine whether Sema3a was expressed in the SA node and the AV node, HCN4 and RFP were co-stained and it was found that Sema3a was not expressed in the SA node and was expressed in a small amount in the AV node (Fig. 5e-f). Cx40 was used as a ventricular conduction system (VCS) marker in the study, so we generated Sema3a CreERT2; R26-tdTomato; Cx40 GFP triple positive mice to detect Sema3a expression in Purkinje fibers. RFP was found to co-localize with the GFP+ cells in the subendocardium of the ventricular wall, whereas the GFP+ coronary artery in the dense myocardium did not detect the RFP signal (Fig. 5g). Overall cardiac fluorescence results showed that Cx40 showed overall expression in the atria, while almost no Sema3a was detected. Cx40 is also expressed in the His (HB) bundle in the left and right bundles and in the Purkinje fibers, which is consistent with previous reports. Compared to Cx40 (GFP signal), Sema3a (RFP signal) is largely only present in Purkinje fibers and is not detected in His bundles or bundles (Fig. 5h-i). Z-stack confocal confirmed that Sema3a (RFP signal) was expressed in Cx40-positive Purkinje fibers in the ventricular myocardium (Fig. 5j). These data indicate that Sema3a-CreERT2 is capable of specifically targeting ventricular Purkinje fibers in the adult mouse heart.
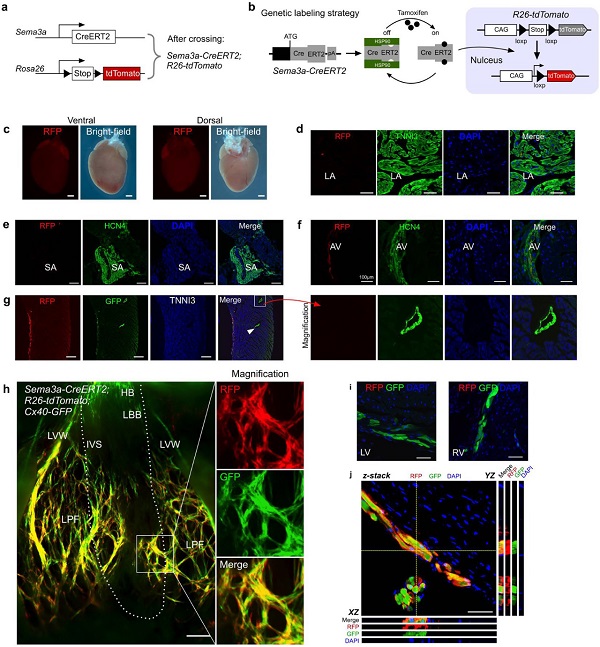
Figure 5. Sema3a expression profile in the heart of adult mice. (a) Sema3a-CreERT2; R26-tdTomato mouse mating schematic. (b) Schematic representation of marker Sema3a+ cells induced by tamoxifen. (c) Sema3a-CreERT2; Schematic diagram of the overall red fluorescent RFP expression in the heart of R26-tdTomato mice. (d) Sema3a-CreERT2; RFP and TNNI3 immunofluorescence staining of R26-tdTomato mice showed rare RFP+ cells in the atria. (e) No Sema3a+ cells were detected in the SA node. (f) Expression of Sema3a in the AV node. (g) Sema3a-CreERT2; Immunostaining of RFP, GFP and TNNI3 in R26-tdTomato mice showed that the CX40+ coronary artery (shown by the arrow) was RFP negative. (h) Sema3a-CreERT2; R26-tdTomato; overall fluorescence view of Cx40-GFP mouse hearts. The dashed line indicates the boundary between IVS and LVW. (i) Sema3a was not detected in Sema3a-CreERT2; R26-tdTomato; Cx40-GFP mouse heart LBB or RBB, while Cx40-GFP was positive. (j) Z-stack confocal display shows Sema3a expression in Purkinje fibers.
Pedigree tracking of Purkinje fibers using Sema3a-CreERT2.
Since Sema3a is more widely expressed in embryonic than in adulthood, the question is, when did Sema3a+ cardiomyocytes begin to develop into the fate of Purkinje fiber?
Tamoxifen was injected into E12.5, E14.5 or E18.5 days of Sema3a-CreERT2; R26-tdTomato; Cx40-GFP triple positive mice, and mouse hearts were analyzed on P7 or P21 days. Lineage tracer data showed that E12.5 began to induce labeled Sema3a+ cells producing Cx40-positive Purkinje fibers (small) and Cx40-negative working cardiomyocytes (mostly) at P7 and P21 (Fig. 6a-b). The Sema3a+ cells of E14.5 were more restricted to the endocardial layer of the ventricular myocardium and produced Cx40-positive Purkinje fibers and Cx40-negative working cardiomyocytes at P7 and P21 (Fig. 6c-d). E18.5 began to induce marker-labeled Sema3a+ cells producing almost all Cx40-positive Purkinje fibers on the P7 and P21 ventricle subendothelial surfaces, while Cx40-negative working cardiomyocytes were rare (Fig. 6e-f). . These data indicate that Sema3a+ cardiomyocytes begin their ventricular conduction system specialization fate during the perinatal phase (eg, E18.5 days) (Fig. 6g).

Figure 6. Transmission system specialization of Sema3a+ cardiomyocytes during cardiac development.
(af) Administration of tamoxifen to Sema3a-CreERT2; R26-tdTomato; Cx40-GFP triple positive mice at E12.5 (a, b), E14.5 (c, d) and E18.5 (e, f) Fen induction. Hearts were collected in each group at P7 and P21. Z-stack confocal showed the fluorescence expression of Sema3a and Cx40. (g) Schematic representation of Sema3a+ cells (red) and Cx40+ cells (green) in developing and adult hearts, respectively.
Quantification of Sema3a+ cardiomyocytes.
What is the proportion of Sema3a+ cardiomyocytes in the heart? The Sema3a+ marker was induced by injecting tamoxifen into adult mice in vivo, and the heart was analyzed 48 hours after tamoxifen treatment. RFP and TNNI3 immunofluorescence staining showed RFP+ cardiomyocytes on the subendocardial surface of the ventricular myocardium (Fig. 7a). Quantitative analysis of the proportion of RFP+ cardiomyocytes in heart sections showed that 1.02±0.13% of cardiomyocytes were marked with red fluorescence by Sema3a-CreERT2, whereas in the absence of tamoxifen, almost no cardiomyocytes were labeled (Fig. 7c) . Separation of cardiomyocytes for quantification (Fig. 7b, d) also yielded consistent results.
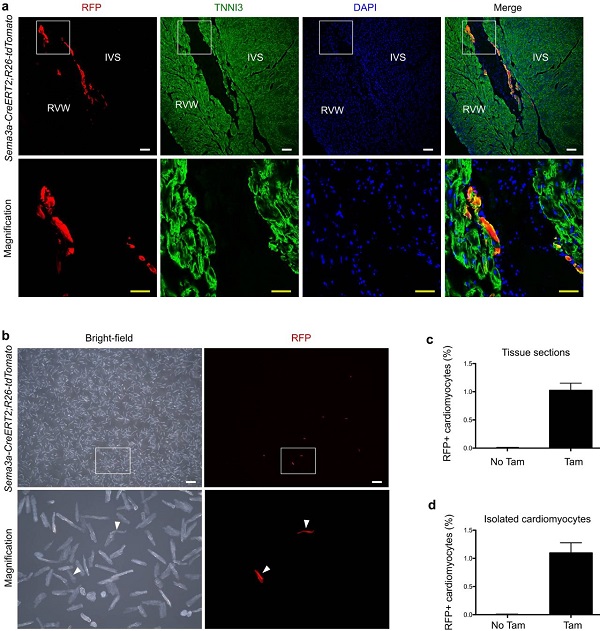
Figure 7. Quantitative analysis of the number of Sema3a+ cardiomyocytes in adult hearts.
(a) Immunofluorescence staining of RFP and TNNI3 in adult hearts. IVS, ventricular septum; RVW, right ventricular wall. (b) Isolated cardiomyocytes from adult Sema3a CreERT2; R26-tdtomato mice. (c) Quantification of the percentage of cardiac RFP + cardiomyocytes. (d) Percentage of RFP+ cardiomyocytes after quantitative isolation.
Sema3a-CreERT2 is a novel genetic tool for mice that specifically target Purkinje fibers in the heart of adult mice. It is compared to Hcn4-CreERT2 (targeting sinoatrial node, atrioventricular node, His bundle, bundle branch and Purkinje fiber) and Cntn2-Cre (targeting SA knot, AV knot and His-Purkinje system) to Purkinje fiber More targeted. Sema3a-CreERT2 also does not label coronary endothelial cells or atrial cardiomyocytes compared to the Cx40-CreERT2 tool.
The lineage tracing results of Purkinje fiber showed that Sema3a+ cells mainly developed into trabecular myocytes in the early embryonic stage, while during the perinatal period, Sema3a+ cardiomyocytes showed the fate of Purkinje fibers.
Original link
Surgical Gowns,Medical Surgical Gown,Isolation Surgical Gown,Disposable Surgical Isolation Gown
Xinxiang Huaxi Sanitary Materials Co., Ltd. , https://www.huaximedical.com