While the lung cells exposed the gas-liquid interface to simulate the real conditions in vivo, it was found that this method may have a serious problem: the cell survival rate of the clean air control group was significantly lower than that of the cells in the incubator. The cell viability in the incubator is 40% to 60% , as shown in Figure 1 below . The emergence of this problem means that the cause of cell damage in the experimental group is more complicated, and the significance of the obtained experimental data is reduced.
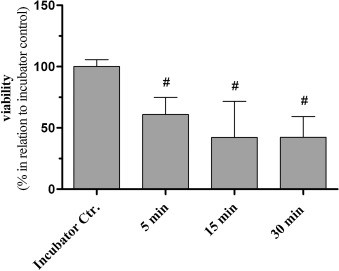
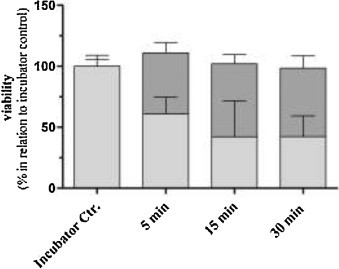
Figure 1 Figure 2
In order to improve the in vitro cell gas-liquid interface exposure method, the German CULTEX® Laboratories experimenter conducted a large number of improved experiments through the CULTEX exposure module structure, cell culture selection, gas path design and airflow control, experimental operation flow and other aspects ( Since the methods and data used in the optimization scheme are all based on a large number of experiments based on the size and pneumatic design of the CULTEX RFS, this scheme is only applicable to the CULTEX RFS system for adjustment and optimization:
Experimental material: Human lung adenocarcinoma epithelial cell line A549 (CCL-185, ATCC) was used for the exposure experiment. A549 cells were grown in Dulbecco's Modified Eagle's Medium (DMEM) (Biochrom, Germany) containing 10% fetal bovine serum (FCS Gold, PAA Laboratories, Germany) and 1% penicillin/streptomycin (Gibco Life Technologies, Germany).
In the experiment, A549 cells were seeded in BD Falcon (PET membrane, 4.2 cm2, pore size 0.4 μm, pore density 2.0 ± 0.2 × 106, BD Falcon, Germany) or Corning Transwell (PET membrane, 4.67 cm2, pore size 0.4 μm, pore density 4.0 × 106, Corning, Germany) Cell chambers were cultured for 24 h at a density of 1.2 × 105 cells/cm 2 under conditions of 37 ° C and 5% CO 2 (standard conditions).
Optimization:
1. Optimization of main structure of CULTEX RFS module
1 The CULTEX RFS module is the core structure for cell culture and exposure in vitro. Compared with the first generation linear CLUTEX module, the RFS module uses a Radial Flow System structure, which not only allows the deposition of particulate aerosols on the cell surface. Uniform, more repeatable. At the same time, the structure is more synchronous and uniform in the distribution of the intake air flow, the release of the overload air flow, and the exhaust gas flow, so as to facilitate the uniform control of the air flow pressure;
2 The system optimizes the structural design of the pre-discharge channel of the overload gas. This design minimizes the influence of the system overload gas discharge on the pressure in the chamber, and at the same time discharges the impurities that may be mixed in the gas to reduce the influence of the polluted air on the cells; Eq \o\ac(â—‹,3)3 gas nozzles can be replaced. According to different brands of tranwell chambers, CULTEX has developed a gas nozzle suitable for use, reducing the gas caused by nozzles and chambers. Pressure; eq \o\ac(â—‹,4)4 The opening and closing of RFS exposure can be completed only by rotating the knob. It not only makes the operation of the system simpler and faster, but more importantly, its airtightness is more intact. There is no accidental effect on the cells due to air leaks.
2. Optimization of gas flow control design for CULTEX RFS system
The airflow control of the CULTEX RFS system is carried out by a vacuum + mass flow controller. 1 CULTEX engineers add two airflow buffer structures to the exhaust gas treatment end; 2 optimize the optimal chamber gas flow; 3 optimize the optimal ventilation pressure .
3, CULTEX RFS module operation process optimization
1 Optimize the effect of the opening and closing of the CULTEX RFS module; eq \o\ac(○,2)2 The medium at the bottom of the membrane is the most important factor affecting the cell, optimizing its liquid level; eq \o\ac (○, 3) 3 Optimization module cleaning method and autoclave effect. After repeated trials, CULTEX® Laboratories has been optimized from the above three points and provides a mature standard operating procedure.
4, cell culture chamber selection
The cell culture chamber is a key site for cell growth and aerosol exposure. CULTEX® Laboratories found that the cell activity in the BD Falcon chamber was consistent with the persistence of exposure time, although the cell activity in the clean air exposed group remained stable. Morphological irregularities, especially in the central location of the chamber, are more pronounced after 30 minutes of clean air exposure (Figure 3 A below). To this end, CULTEX® Laboratories sought and developed the best chamber for RFS use. After 30 minutes of clean air exposure (Figure 3 below), the cells were evenly distributed and the cell viability was not affected.
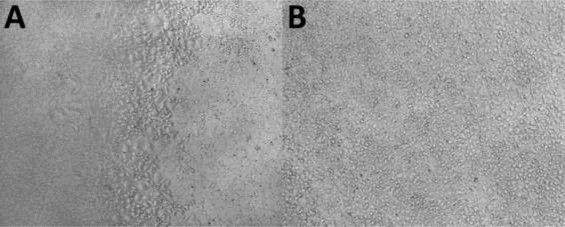
image 3
In summary, a series of optimized results from CULTEX® Laboratories for Cultex RFS showed that the effect of cell viability in the clean air control group was observed in vitro , but it could be reduced by a number of optimized treatment experiments. The optimized Cultex RFS system for A549 cell exposure to clean air also proves that Cultex RFS technology is a good alternative method and an important in vitro model of pulmonary cytotoxic cells, which is of great significance for the development of inhalation toxicology.
Note: The data and data in this article are from CULTEX® Laboratorie GmbH. For more information and data on CULTEX cell in vitro exposure methods and optimization schemes, please contact CULTEX® Laboratorie in Germany or Beijing Lelecheng Technology Co., Ltd., China Technical Service Center.
Eventually, the cell viability in the CULTEX RFS cell exposure module was kept essentially the same as in the incubator control group (see Figure 2 above).
Head Massage,Head Massage Spa,Best Head Massager,Relaxing Head Massage
Shenzhen Jie Zhong Lian Investment Co., Ltd. , https://www.szmeizons.com